A tagging-via-substrate technology for detection and proteomics of farnesylated proteins
- PMID: 15308774
- PMCID: PMC515085
- DOI: 10.1073/pnas.0403413101
A tagging-via-substrate technology for detection and proteomics of farnesylated proteins
Abstract
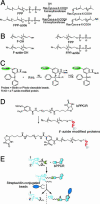
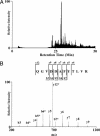
A recently developed proteomics strategy, designated tagging-via-substrate (TAS) approach, is described for the detection and proteomic analysis of farnesylated proteins. TAS technology involves metabolic incorporation of a synthetic azido-farnesyl analog and chemoselective derivatization of azido-farnesyl-modified proteins by an elegant version of Staudinger reaction, pioneered by the Bertozzi group, using a biotinylated phosphine capture reagent. The resulting protein conjugates can be specifically detected and/or affinity-purified by streptavidin-linked horseradish peroxidase or agarose beads, respectively. Thus, the technology enables global profiling of farnesylated proteins by enriching farnesylated proteins and reducing the complexity of farnesylation subproteome. Azido-farnesylated proteins maintain the properties of protein farnesylation, including promoting membrane association, Ras-dependent mitogen-activated protein kinase kinase activation, and inhibition of lovastatin-induced apoptosis. A proteomic analysis of farnesylated proteins by TAS technology revealed 18 farnesylated proteins, including those with potentially novel farnesylation motifs, suggesting that future use of this method is likely to yield novel insight into protein farnesylation. TAS technology can be extended to other posttranslational modifications, such as geranylgeranylation and myristoylation, thus providing powerful tools for detection, quantification, and proteomic analysis of posttranslationally modified proteins.
Figures



Similar articles
-
A tagging-via-substrate technology for genome-wide detection and identification of farnesylated proteins.Methods Enzymol. 2006;407:629-37. doi: 10.1016/S0076-6879(05)07049-7. Methods Enzymol. 2006. PMID: 16757357
-
Tagging-via-substrate strategy for probing O-GlcNAc modified proteins.J Proteome Res. 2005 May-Jun;4(3):950-7. doi: 10.1021/pr050033j. J Proteome Res. 2005. PMID: 15952742
-
A tagging-via-substrate approach to detect the farnesylated proteome using two-dimensional electrophoresis coupled with Western blotting.Mol Cell Proteomics. 2010 Apr;9(4):742-51. doi: 10.1074/mcp.M900597-MCP200. Epub 2010 Jan 26. Mol Cell Proteomics. 2010. PMID: 20103566 Free PMC article.
-
Inhibitors of prenylation of Ras and other G-proteins and their application as therapeutics.Biochem Pharmacol. 2000 Oct 15;60(8):1061-8. doi: 10.1016/s0006-2952(00)00386-5. Biochem Pharmacol. 2000. PMID: 11007942 Review.
-
The balance of protein farnesylation and geranylgeranylation during the progression of nonalcoholic fatty liver disease.J Biol Chem. 2020 Apr 10;295(15):5152-5162. doi: 10.1074/jbc.REV119.008897. Epub 2020 Mar 5. J Biol Chem. 2020. PMID: 32139507 Free PMC article. Review.
Cited by
-
A property-based analysis of human transcription factors.BMC Res Notes. 2015 Mar 14;8:82. doi: 10.1186/s13104-015-1039-6. BMC Res Notes. 2015. PMID: 25890365 Free PMC article.
-
In situ visualization of newly synthesized proteins in environmental microbes using amino acid tagging and click chemistry.Environ Microbiol. 2014 Aug;16(8):2568-90. doi: 10.1111/1462-2920.12436. Epub 2014 Apr 2. Environ Microbiol. 2014. PMID: 24571640 Free PMC article.
-
Click chemistry in complex mixtures: bioorthogonal bioconjugation.Chem Biol. 2014 Sep 18;21(9):1075-101. doi: 10.1016/j.chembiol.2014.09.002. Chem Biol. 2014. PMID: 25237856 Free PMC article. Review.
-
Synthetic isoprenoid analogues for the study of prenylated proteins: Fluorescent imaging and proteomic applications.Bioorg Chem. 2016 Feb;64:59-65. doi: 10.1016/j.bioorg.2015.12.003. Epub 2015 Dec 10. Bioorg Chem. 2016. PMID: 26709869 Free PMC article. Review.
-
Synthetic Biology-Empowered Hydrogels for Medical Diagnostics.Adv Biochem Eng Biotechnol. 2021;178:197-226. doi: 10.1007/10_2020_158. Adv Biochem Eng Biotechnol. 2021. PMID: 33582837
References
-
- Gudepu, R. G. & Wold, F. (1998) in Proteins: Analysis and Design, ed. Angeletti, R. H. (Academic, San Diego), pp. 121–207.
-
- Reiss, Y., Goldstein, J. L., Seabra, M. C., Casey, P. J. & Brown, M. S. (1990) Cell 62, 81–88. - PubMed
-
- Moores, S. L., Schaber, M. D., Mosser, S. D., Rands, E., O'Hara, M. B., Garsky, V. M., Marshall, M. S., Pompliano, D. L. & Gibbs, J. B. (1991) J. Biol. Chem. 266, 14603–14610. - PubMed
-
- Seabra, M. C., Reiss, Y., Casey, P. J., Brown, M. S. & Goldstein, J. L. (1991) Cell 65, 429–434. - PubMed
-
- Spence, R. A. & Casey, P. J. (2000) Mechanism of Catalysis by Protein Farnesyltransferase (Academic, San Diego).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

