Capecitabine and vinorelbine in patients with metastatic breast cancer previously treated with anthracycline and taxane
- PMID: 15308846
- PMCID: PMC2816889
- DOI: 10.3346/jkms.2004.19.4.547
Capecitabine and vinorelbine in patients with metastatic breast cancer previously treated with anthracycline and taxane
Abstract
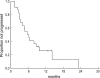
We have evaluated the efficacy and safety of the combination of capecitabine and vinorelbine in metastatic breast cancer (MBC) patients previously treated with anthracycline-and taxane-containing regimens. Between April 2000 and September 2002, 44 female MBC patients received oral capecitabine (1,250 mg/m2 twice daily on days 114), and intravenous vinorelbine (25 mg/m2 on days 1 and 8) during each 3 week-chemotherapy cycle (median, 5 cycles/patient; total, 235 cycles). One patient achieved a complete response and 21 patients had partial responses, giving an overall response rate of 50% in the intention-to-treat analysis (95% CI, 35.0-65.0%). Median duration of response was 6.0 months (range 1.2-23.0 months). Patients were followed- up for a median of 16 months, with median progression-free survival being 5.3 months, and median overall survival being 17 months. Toxicities included grades III and IV neutropenia in 63 (26.8%) and 4 (1.7%) cycles, respectively, and grades II and III hand-foot syndrome in 12 (5.1%) and 4 (1.7%) cycles, respectively. Other nonhematologic toxicities were minimal and manageable. In conclusion, the combination of capecitabine and vinorelbine was effective and well tolerated in MBC patients even after treatment with anthracyclines and taxanes.
Copyright The Korean Academy of Medical Sciences
Figures
References
-
- Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. - PubMed
-
- Tormey C. Adriamycin (NSC 123-127) in breast cancer. An overview of studies. Cancer Chemother Rep. 1975;3:319–327.
-
- Stewart DJ, Evans WK, Shepherd FA, Wilson KS, Pritchard KI, Trudeau ME, Wilson JJ, Martz K. Cyclophosphamide and fluorouracil combined with mitoxantrone versus doxorubicin for breast cancer: Superiority of doxorubicin. J Clin Oncol. 1997;15:1897–1905. - PubMed
-
- Perez EA. Paclitaxel in breast cancer. Oncologist. 1998;3:373–389. - PubMed
-
- Budman DR. Vinorelbine (Navelbine): A third-generation vinca alkaloid. Cancer Invest. 1997;15:475–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous