Functional characterization of nine Norway Spruce TPS genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily
- PMID: 15310829
- PMCID: PMC520763
- DOI: 10.1104/pp.104.042028
Functional characterization of nine Norway Spruce TPS genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily
Abstract
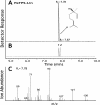
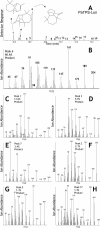
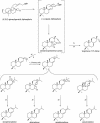
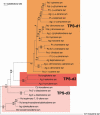
Constitutive and induced terpenoids are important defense compounds for many plants against potential herbivores and pathogens. In Norway spruce (Picea abies L. Karst), treatment with methyl jasmonate induces complex chemical and biochemical terpenoid defense responses associated with traumatic resin duct development in stems and volatile terpenoid emissions in needles. The cloning of (+)-3-carene synthase was the first step in characterizing this system at the molecular genetic level. Here we report the isolation and functional characterization of nine additional terpene synthase (TPS) cDNAs from Norway spruce. These cDNAs encode four monoterpene synthases, myrcene synthase, (-)-limonene synthase, (-)-alpha/beta-pinene synthase, and (-)-linalool synthase; three sesquiterpene synthases, longifolene synthase, E,E-alpha-farnesene synthase, and E-alpha-bisabolene synthase; and two diterpene synthases, isopimara-7,15-diene synthase and levopimaradiene/abietadiene synthase, each with a unique product profile. To our knowledge, genes encoding isopimara-7,15-diene synthase and longifolene synthase have not been previously described, and this linalool synthase is the first described from a gymnosperm. These functionally diverse TPS account for much of the structural diversity of constitutive and methyl jasmonate-induced terpenoids in foliage, xylem, bark, and volatile emissions from needles of Norway spruce. Phylogenetic analyses based on the inclusion of these TPS into the TPS-d subfamily revealed that functional specialization of conifer TPS occurred before speciation of Pinaceae. Furthermore, based on TPS enclaves created by distinct branching patterns, the TPS-d subfamily is divided into three groups according to sequence similarities and functional assessment. Similarities of TPS evolution in angiosperms and modeling of TPS protein structures are discussed.
Figures




Similar articles
-
Traumatic resin defense in Norway spruce (Picea abies): methyl jasmonate-induced terpene synthase gene expression, and cDNA cloning and functional characterization of (+)-3-carene synthase.Plant Mol Biol. 2003 Jan;51(1):119-33. doi: 10.1023/a:1020714403780. Plant Mol Biol. 2003. PMID: 12602896
-
Wound-induced terpene synthase gene expression in Sitka spruce that exhibit resistance or susceptibility to attack by the white pine weevil.Plant Physiol. 2006 Mar;140(3):1009-21. doi: 10.1104/pp.105.071803. Epub 2006 Jan 13. Plant Physiol. 2006. PMID: 16415217 Free PMC article.
-
Transcriptome mining, functional characterization, and phylogeny of a large terpene synthase gene family in spruce (Picea spp.).BMC Plant Biol. 2011 Mar 7;11:43. doi: 10.1186/1471-2229-11-43. BMC Plant Biol. 2011. PMID: 21385377 Free PMC article.
-
Diversity and variability of terpenoid defences in conifers: molecular genetics, biochemistry and evolution of the terpene synthase gene family in grand fir (Abies grandis).Novartis Found Symp. 1999;223:132-45; discussion 146-9. doi: 10.1002/9780470515679.ch9. Novartis Found Symp. 1999. PMID: 10549552 Review.
-
On the Evolution and Functional Diversity of Terpene Synthases in the Pinus Species: A Review.J Mol Evol. 2020 Apr;88(3):253-283. doi: 10.1007/s00239-020-09930-8. Epub 2020 Feb 8. J Mol Evol. 2020. PMID: 32036402 Review.
Cited by
-
Terpene Specialized Metabolism in Arabidopsis thaliana.Arabidopsis Book. 2011;9:e0143. doi: 10.1199/tab.0143. Epub 2011 Apr 6. Arabidopsis Book. 2011. PMID: 22303268 Free PMC article.
-
Insights into diterpene cyclization from structure of bifunctional abietadiene synthase from Abies grandis.J Biol Chem. 2012 Feb 24;287(9):6840-50. doi: 10.1074/jbc.M111.337592. Epub 2012 Jan 4. J Biol Chem. 2012. PMID: 22219188 Free PMC article.
-
Assessment and implications of intraspecific and phenological variability in monoterpenes of Scots pine (Pinus sylvestris) foliage.J Chem Ecol. 2007 Mar;33(3):477-91. doi: 10.1007/s10886-006-9244-3. J Chem Ecol. 2007. PMID: 17268824
-
Dual RNA-seq analysis provides new insights into interactions between Norway spruce and necrotrophic pathogen Heterobasidion annosum s.l.BMC Plant Biol. 2019 Jan 3;19(1):2. doi: 10.1186/s12870-018-1602-0. BMC Plant Biol. 2019. PMID: 30606115 Free PMC article.
-
Volatiles from a mite-infested spruce clone and their effects on pine weevil behavior.J Chem Ecol. 2009 Oct;35(10):1262-71. doi: 10.1007/s10886-009-9708-3. Epub 2009 Nov 10. J Chem Ecol. 2009. PMID: 19902304
References
-
- Adams RP (2002) The Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Allured Publishing, Carol Stream, IL
-
- Alfaro RI, Borden JH, King JN, Tomlin ES, McIntosh RL, Bohlmann J (2002) Mechanisms of resistance in conifers against shoot infesting insects. In MR Wagner, KM Clancy, F Lieutier, TD Paine, eds, Mechanisms and Deployment of Resistance in Trees to Insects. Kluwer Academic Press, Dordrecht, The Netherlands, pp 101–126
-
- Arimura G, Huber DPW, Bohlmann J (2004) Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa x deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (−)-germacrene D synthase, PtdTPS1. Plant J 37: 603–616 - PubMed
-
- Aubourg S, Lecharny A, Bohlmann J (2002) Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol Genet Genomics 267: 730–745 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

