Dynamic changes in the osmotic water permeability of protoplast plasma membrane
- PMID: 15310831
- PMCID: PMC520799
- DOI: 10.1104/pp.104.043000
Dynamic changes in the osmotic water permeability of protoplast plasma membrane
Abstract
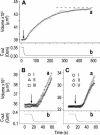
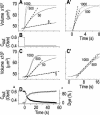
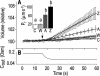
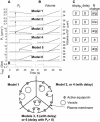
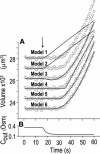
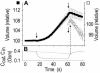
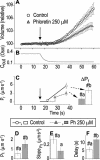
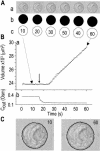
The osmotic water permeability coefficient (P(f)) of plasma membrane of maize (Zea mays) Black Mexican Sweet protoplasts changed dynamically during a hypoosmotic challenge, as revealed using a model-based computational approach. The best-fitting model had three free parameters: initial P(f), P(f) rate-of-change (slope(P(f))), and a delay, which were hypothesized to reflect changes in the number and/or activity of aquaporins in the plasma membrane. Remarkably, the swelling response was delayed 2 to 11 s after start of the noninstantaneous (but accounted for) bath flush. The P(f) during the delay was < or =1 microm s(-1). During the swelling period following the delay, P(f) changed dynamically: within the first 15 s P(f) either (1) increased gradually to approximately 8 microm s(-1) (in the majority population of low-initial-P(f) cells) or (2) increased abruptly to 10 to 20 microm s(-1) and then decreased gradually to 3 to 6 microm s(-1) (in the minority population of high-initial-P(f) cells). We affirmed the validity of our computational approach by the ability to reproduce previously reported initial P(f) values (including the absence of delay) in control experiments on Xenopus oocytes expressing the maize aquaporin ZmPIP2;5. Although mercury did not affect the P(f) in swelling Black Mexican Sweet cells, phloretin, another aquaporin inhibitor, inhibited swelling in a predicted manner, prolonging the delay and slowing P(f) increase, thereby confirming the hypothesis that P(f) dynamics, delay included, reflected the varying activity of aquaporins.
Figures
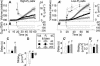
References
-
- Bick I, Thiel G, Homann U (2001) Cytochalasin D attenuates the desensitisation of pressure-stimulated vesicle fusion in guard cell protoplasts. Eur J Cell Biol 80: 521–526 - PubMed
-
- Brown D (2003) The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284: F893–F901 - PubMed
-
- Cosgrove DJ, Hedrich R (1991) Stretch-activated chloride, potassium, and calcium channels coexisting in plasma membranes of guard cells of Vicia faba L Planta 186: 143–153 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

