Derivation of midbrain dopamine neurons from human embryonic stem cells
- PMID: 15310843
- PMCID: PMC515094
- DOI: 10.1073/pnas.0404700101
Derivation of midbrain dopamine neurons from human embryonic stem cells
Abstract
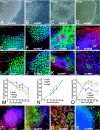
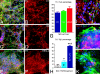
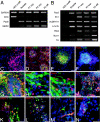
Human embryonic stem (hES) cells are defined by their extensive self-renewal capacity and their potential to differentiate into any cell type of the human body. The challenge in using hES cells for developmental biology and regenerative medicine has been to direct the wide differentiation potential toward the derivation of a specific cell fate. Within the nervous system, hES cells have been shown to differentiate in vitro into neural progenitor cells, neurons, and astrocytes. However, to our knowledge, the selective derivation of any given neuron subtype has not yet been demonstrated. Here, we describe conditions to direct hES cells into neurons of midbrain dopaminergic identity. Neuroectodermal differentiation was triggered on stromal feeder cells followed by regional specification by means of the sequential application of defined patterning molecules that direct in vivo midbrain development. Progression toward a midbrain dopamine (DA) neuron fate was monitored by the sequential expression of key transcription factors, including Pax2, Pax5, and engrailed-1 (En1), measurements of DA release, the presence of tetrodotoxin-sensitive action potentials, and the electron-microscopic visualization of tyrosinehydroxylase-positive synaptic terminals. High-yield DA neuron derivation was confirmed from three independent hES and two monkey embryonic stem cell lines. The availability of unlimited numbers of midbrain DA neurons is a first step toward exploring the potential of hES cells in preclinical models of Parkinson's disease. This experimental system also provides a powerful tool to probe the molecular mechanisms that control the development and function of human midbrain DA neurons.
Figures


References
-
- Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S. & Jones, J. M. (1998) Science 282, 1145–1147. - PubMed
-
- Lee, S.-H., Lumelsky, N., Studer, L., Auerbach, J. M. & McKay, R. D. G. (2000) Nat. Biotechnol. 18, 675–679. - PubMed
-
- Kawasaki, H., Mizuseki, K., Nishikawa, S., Kaneko, S., Kuwana, Y., Nakanishi, S., Nishikawa, S. I. & Sasai, Y. (2000) Neuron 28, 31–40. - PubMed
-
- Wichterle, H., Lieberam, I., Porter, J. A. & Jessell, T. M. (2002) Cell 110, 385–397. - PubMed
-
- Brustle, O., Jones, K. N., Learish, R. D., Karram, K., Choudhary, K., Wiestler, O. D., Duncan, I. D. & McKay, R. G. (1999) Science 285, 754–756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

