Vaccination with genetically engineered allergens prevents progression of allergic disease
- PMID: 15310844
- PMCID: PMC521981
- DOI: 10.1073/pnas.0404735101
Vaccination with genetically engineered allergens prevents progression of allergic disease
Abstract
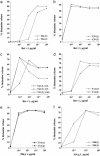
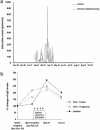
IgE-mediated allergy affects >25% of the population in industrialized countries. Repeated contact with the disease-eliciting allergens induces rises of allergen-specific IgE Abs and progression of the disease to more severe manifestations. Our study uses a type of vaccine that is based on genetically modified allergen derivatives to treat allergic patients. We developed hypoallergenic derivatives of the major birch pollen allergen, Bet v 1, by genetic engineering and vaccinated birch pollen-allergic patients (n = 124) in a double-blind, placebo-controlled study. Active treatment induced protective IgG Abs that inhibited allergen-induced release of inflammatory mediators. We also observed a reduction of cutaneous sensitivity as well as an improvement of symptoms in actively treated patients. Most important, rises of allergen-specific IgE induced by seasonal birch pollen exposure were significantly reduced in vaccinated patients. Vaccination with genetically engineered allergen derivatives is a therapy for allergy that not only ameliorates allergic reactions but also reduces the IgE production underlying the disease.
Figures


References
-
- Kay, A. B. (1997) Allergy and Allergic Diseases (Blackwell Science, Oxford).
-
- Wills-Karp, M., Santeliz, M. & Karp, C. L. (2001) Nat. Rev. Immunol. 1, 69-75. - PubMed
-
- Valenta, R. (2002) Nat. Rev. Immunol. 2, 446-453. - PubMed
-
- Kinet, J. P. (1999) Annu. Rev. Immunol. 17, 931-972. - PubMed
-
- Hendersen, L. L., Larson, J. B. & Gleich, G. J. (1975) J. Allergy Clin. Immunol. 55, 10-15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

