Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia
- PMID: 15310849
- PMCID: PMC515104
- DOI: 10.1073/pnas.0405077101
Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia
Abstract
GRM3, a metabotropic glutamate receptor-modulating synaptic glutamate, is a promising schizophrenia candidate gene. In a family-based association study, a common GRM3 haplotype was strongly associated with schizophrenia (P = 0.0001). Within this haplotype, the A allele of single-nucleotide polymorphism (SNP) 4 (hCV11245618) in intron 2 was slightly overtransmitted to probands (P = 0.02). We studied the effects of this SNP on neurobiological traits related to risk for schizophrenia and glutamate neurotransmission. The SNP4 A allele was associated with poorer performance on several cognitive tests of prefrontal and hippocampal function. The physiological basis of this effect was assessed with functional MRI, which showed relatively deleterious activation patterns in both cortical regions in control subjects homozygous for the SNP4 A allele. We next looked at SNP4's effects on two indirect measures of prefrontal glutamate neurotransmission. Prefrontal N-acetylaspartate, an in vivo MRI measure related to synaptic activity and closely correlated with tissue glutamate, was lower in SNP4 AA homozygotes. In postmortem human prefrontal cortex, AA homozygotes had lower mRNA levels of the glial glutamate transporter EAAT2, a protein regulated by GRM3 that critically modulates synaptic glutamate. Effects of SNP4 on prefrontal GRM3 mRNA and protein levels were marginal. Resequencing revealed no missense or splice-site SNPs, suggesting that the intronic SNP4 or related haplotypes may exert subtle regulatory effects on GRM3 transcription. These convergent data point to a specific molecular pathway by which GRM3 genotype alters glutamate neurotransmission, prefrontal and hippocampal physiology and cognition, and thereby increased risk for schizophrenia.
Figures
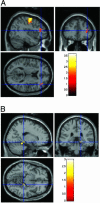

References
-
- Weinberger, D. R., Egan, M. F., Bertolino, A., Callicott, J. H., Mattay, V. S., Lipska, B. K., Berman, K. F. & Goldberg, T. E. (2001) Biol. Psychiatry 50, 825–844. - PubMed
-
- Goldberg, T. E. & Weinberger, D. R. (1988) Schizophr. Bull. 14, 179–183. - PubMed
-
- Callicott, J. H., Bertolino, A., Mattay, V. S., Langheim, F. J. P., Duyn, J., Coppola, R., Goldberg, T. E. & Weinberger, D. R. (2000) Cereb. Cortex 10, 1078–1092. - PubMed
-
- Selemon, L. D. & Goldman-Rakic, P. S. (1999) Biol. Psychiatry 45, 17–25. - PubMed
-
- Deakin, J. F., Slater, P., Simpson, M. D., Gilchrist, A. C., Skan, W. J., Royston, M. C., Reynolds, G. P. & Cross, A. J. (1989) J. Neurochem. 52, 1781–1786. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

