Interactions among p22, glyceraldehyde-3-phosphate dehydrogenase and microtubules
- PMID: 15312048
- PMCID: PMC1134116
- DOI: 10.1042/BJ20040622
Interactions among p22, glyceraldehyde-3-phosphate dehydrogenase and microtubules
Abstract
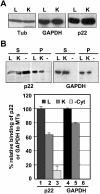
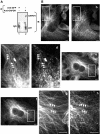
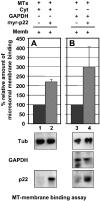
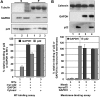
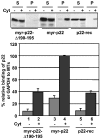
Previously, we have shown that p22, an EF-hand Ca2+-binding protein, interacts indirectly with microtubules in an N-myristoylation-dependent and Ca2+-independent manner. In the present study, we report that N-myristoylated p22 interacts with several microtubule-associated proteins within the 30-100 kDa range using overlay blots of microtubule pellets containing cytosolic proteins. One of those p22-binding partners, a 35-40 kDa microtubule-binding protein, has been identified by MS as GAPDH (glyceraldehyde-3-phosphate dehydrogenase). Several lines of evidence suggest a functional relationship between GAPDH and p22. First, endogenous p22 interacts with GAPDH by immunoprecipitation. Secondly, p22 and GAPDH align along microtubule tracks in analogous punctate structures in BHK cells. Thirdly, GAPDH facilitates the p22-dependent interactions between microtubules and microsomal membranes, by increasing the ability of p22 to bind microtubules but not membranes. We have also shown a direct interaction between N-myristoylated p22 and GAPDH in vitro with a K(D) of approximately 0.5 microM. The removal of either the N-myristoyl group or the last six C-terminal amino acids abolishes the binding of p22 to GAPDH and reduces the ability of p22 to associate with microtubules. In summary, we report that GAPDH is involved in the ability of p22 to facilitate microtubule-membrane interactions by affecting the p22-microtubule, but not the p22-membrane, association.
Figures



References
-
- Allan V. J., Schroer T. A. Membrane motors. Curr. Opin. Cell Biol. 1999;11:476–482. - PubMed
-
- Karcher R. L., Deacon S. W., Gelfand V. I. Motor-cargo interactions: the key to transport specificity. Trends Cell Biol. 2002;12:21–27. - PubMed
-
- Cassimeris L., Spittle C. Regulation of microtubule-associated proteins. Int. Rev. Cytol. 2001;210:163–226. - PubMed
-
- Schuyler S. C., Pellman D. Microtubule ‘plus-end-tracking proteins’: the end is just the beginning. Cell (Cambridge, Mass.) 2001;105:421–424. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

