Brazilian Sepsis Epidemiological Study (BASES study)
- PMID: 15312226
- PMCID: PMC522852
- DOI: 10.1186/cc2892
Brazilian Sepsis Epidemiological Study (BASES study)
Abstract
Introduction: Consistent data about the incidence and outcome of sepsis in Latin American intensive care units (ICUs), including Brazil, are lacking. This study was designed to verify the actual incidence density and outcome of sepsis in Brazilian ICUs. We also assessed the association between the Consensus Conference criteria and outcome
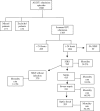
Methods: This is a multicenter observational cohort study performed in five private and public, mixed ICUs from two different regions of Brazil. We prospectively followed 1383 adult patients consecutively admitted to those ICUs from May 2001 to January 2002, until their discharge, 28th day of stay, or death. For all patients we collected the following data at ICU admission: age, gender, hospital and ICU admission diagnosis, APACHE II score, and associated underlying diseases. During the following days, we looked for systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock criteria, as well as recording the sequential organ failure assessment score. Infection was diagnosed according to CDC criteria for nosocomial infection, and for community-acquired infection, clinical, radiological and microbiological parameters were used.
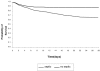
Results: For the whole cohort, median age was 65.2 years (49-76), median length of stay was 2 days (1-6), and the overall 28-day mortality rate was 21.8%. Considering 1383 patients, the incidence density rates for sepsis, severe sepsis and septic shock were 61.4, 35.6 and 30.0 per 1000 patient-days, respectively. The mortality rate of patients with SIRS, sepsis, severe sepsis and septic shock increased progressively from 24.3% to 34.7%, 47.3% and 52.2%, respectively. For patients with SIRS without infection the mortality rate was 11.3%. The main source of infection was lung/respiratory tract.
Conclusion: Our preliminary data suggest that sepsis is a major public health problem in Brazilian ICUs, with an incidence density about 57 per 1000 patient-days. Moreover, there was a close association between ACCP/SCCM categories and mortality rate.
Figures
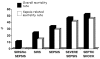
Comment in
-
Severe sepsis epidemiology: sampling, selection, and society.Crit Care. 2004 Aug;8(4):222-6. doi: 10.1186/cc2917. Epub 2004 Jul 9. Crit Care. 2004. PMID: 15312201 Free PMC article.
References
-
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Recombinant human Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

