A subcutaneously injected UV-inactivated SARS coronavirus vaccine elicits systemic humoral immunity in mice
- PMID: 15314040
- PMCID: PMC7108621
- DOI: 10.1093/intimm/dxh143
A subcutaneously injected UV-inactivated SARS coronavirus vaccine elicits systemic humoral immunity in mice
Abstract
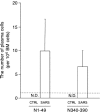
The recent emergence of severe acute respiratory syndrome (SARS) was caused by a novel coronavirus, SARS-CoV. It spread rapidly to many countries and developing a SARS vaccine is now urgently required. In order to study the immunogenicity of UV-inactivated purified SARS-CoV virion as a vaccine candidate, we subcutaneously immunized mice with UV-inactivated SARS-CoV with or without an adjuvant. We chose aluminum hydroxide gel (alum) as an adjuvant, because of its long safety history for human use. We observed that the UV-inactivated SARS-CoV virion elicited a high level of humoral immunity, resulting in the generation of long-term antibody secreting and memory B cells. With the addition of alum to the vaccine formula, serum IgG production was augmented and reached a level similar to that found in hyper-immunized mice, though it was still insufficient to elicit serum IgA antibodies. Notably, the SARS-CoV virion itself was able to induce long-term antibody production even without an adjuvant. Anti-SARS-CoV antibodies elicited in mice recognized both the spike and nucleocapsid proteins of the virus and were able to neutralize the virus. Furthermore, the UV-inactivated virion induced regional lymph node T-cell proliferation and significant levels of cytokine production (IL-2, IL-4, IL-5, IFN-gamma and TNF-alpha) upon restimulation with inactivated SARS-CoV virion in vitro. Thus, a whole killed virion could serve as a candidate antigen for a SARS vaccine to elicit both humoral and cellular immunity.
Figures



References
-
- Drosten, C., Gunther, S., Preiser, W. et al.2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967. - PubMed
-
- Ksiazek, T. G., Erdman, D., Goldsmith, C. S. et al.2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953. - PubMed
-
- Marra, M. A., Jones, S. J., Astell, C. R. et al.2003. The genome sequence of the SARS-associated coronavirus. Science 300:1399. - PubMed
-
- Rota, P. A., Oberste, M. S., Monroe, S. S. et al.2003. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300:1394. - PubMed
-
- Holmes, K. V. and Enjuanes, L. 2003. Virology. The SARS coronavirus: a postgenomic era. Science 300:1377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

