In vivo monitoring of Ca(2+) uptake into mitochondria of mouse skeletal muscle during contraction
- PMID: 15314066
- PMCID: PMC2172216
- DOI: 10.1083/jcb.200403102
In vivo monitoring of Ca(2+) uptake into mitochondria of mouse skeletal muscle during contraction
Abstract
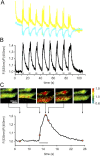
Although the importance of mitochondria in patho-physiology has become increasingly evident, it remains unclear whether these organelles play a role in Ca(2+) handling by skeletal muscle. This undefined situation is mainly due to technical limitations in measuring Ca(2+) transients reliably during the contraction-relaxation cycle. Using two-photon microscopy and genetically expressed "cameleon" Ca(2+) sensors, we developed a robust system that enables the measurement of both cytoplasmic and mitochondrial Ca(2+) transients in vivo. We show here for the first time that, in vivo and under highly physiological conditions, mitochondria in mammalian skeletal muscle take up Ca(2+) during contraction induced by motor nerve stimulation and rapidly release it during relaxation. The mitochondrial Ca(2+) increase is delayed by a few milliseconds compared with the cytosolic Ca(2+) rise and occurs both during a single twitch and upon tetanic contraction.
Figures





References
-
- Arnaudeau, S., W.L. Kelley, J.V. Walsh Jr., and N. Demaurex. 2001. Mitochondria recycle Ca(2+) to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J. Biol. Chem. 276:29430–29439. - PubMed
-
- Bernardi, P. 1999. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 79:1127–1155. - PubMed
-
- Bootman, M.D., P. Lipp, and M.J. Berridge. 2001. The organisation and functions of local Ca(2+) signals. J. Cell Sci. 114:2213–2222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

