Mrp4 confers resistance to topotecan and protects the brain from chemotherapy
- PMID: 15314169
- PMCID: PMC506999
- DOI: 10.1128/MCB.24.17.7612-7621.2004
Mrp4 confers resistance to topotecan and protects the brain from chemotherapy
Abstract
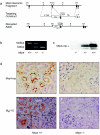
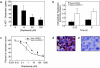
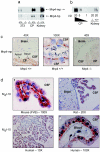
The role of the multidrug resistance protein MRP4/ABCC4 in vivo remains undefined. To explore this role, we generated Mrp4-deficient mice. Unexpectedly, these mice showed enhanced accumulation of the anticancer agent topotecan in brain tissue and cerebrospinal fluid (CSF). Further studies demonstrated that topotecan was an Mrp4 substrate and that cells overexpressing Mrp4 were resistant to its cytotoxic effects. We then used new antibodies to discover that Mrp4 is unique among the anionic ATP-dependent transporters in its dual localization at the basolateral membrane of the choroid plexus epithelium and in the apical membrane of the endothelial cells of the brain capillaries. Microdialysis sampling of ventricular CSF demonstrated that localization of Mrp4 at the choroid epithelium is integral to its function in limiting drug penetration into the CSF. The topotecan resistance of cells overexpressing Mrp4 and the polarized expression of Mrp4 in the choroid plexus and brain capillary endothelial cells indicate that Mrp4 has a dual role in protecting the brain from cytotoxins and suggest that the therapeutic efficacy of central nervous system-directed drugs that are Mrp4 substrates may be improved by developing Mrp4 inhibitors.
Copyright 2004 American Society for Microbiology
Figures
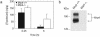

Similar articles
-
Topotecan central nervous system penetration is altered by a tyrosine kinase inhibitor.Cancer Res. 2006 Dec 1;66(23):11305-13. doi: 10.1158/0008-5472.CAN-06-0929. Cancer Res. 2006. PMID: 17145877
-
Topotecan is a substrate for multidrug resistance associated protein 4.Curr Drug Metab. 2006 Jan;7(1):105-18. doi: 10.2174/138920006774832550. Curr Drug Metab. 2006. PMID: 16454695
-
Gender-specific expression of ATP-binding cassette (Abc) transporters and cytoprotective genes in mouse choroid plexus.Toxicology. 2017 Jul 1;386:84-92. doi: 10.1016/j.tox.2017.05.019. Epub 2017 Jun 3. Toxicology. 2017. PMID: 28587784 Free PMC article.
-
Brain drug delivery, drug metabolism, and multidrug resistance at the choroid plexus.Microsc Res Tech. 2001 Jan 1;52(1):83-8. doi: 10.1002/1097-0029(20010101)52:1<83::AID-JEMT10>3.0.CO;2-N. Microsc Res Tech. 2001. PMID: 11135451 Review.
-
MRP4 (ABCC4) as a potential pharmacologic target for cardiovascular disease.Pharmacol Res. 2016 May;107:381-389. doi: 10.1016/j.phrs.2016.04.002. Epub 2016 Apr 5. Pharmacol Res. 2016. PMID: 27063943 Review.
Cited by
-
Choroid Plexus and Drug Removal Mechanisms.AAPS J. 2021 May 3;23(3):61. doi: 10.1208/s12248-021-00587-9. AAPS J. 2021. PMID: 33942198 Review.
-
ABC Transporters at the Blood-Brain Interfaces, Their Study Models, and Drug Delivery Implications in Gliomas.Pharmaceutics. 2019 Dec 23;12(1):20. doi: 10.3390/pharmaceutics12010020. Pharmaceutics. 2019. PMID: 31878061 Free PMC article. Review.
-
A Human ABC Transporter ABCC4 Gene SNP (rs11568658, 559 G > T, G187W) Reduces ABCC4-Dependent Drug Resistance.Cells. 2019 Jan 10;8(1):39. doi: 10.3390/cells8010039. Cells. 2019. PMID: 30634695 Free PMC article.
-
The Role of Biological Rhythms in New Drug Formulations to Cross the Brain Barriers.Int J Mol Sci. 2023 Aug 8;24(16):12541. doi: 10.3390/ijms241612541. Int J Mol Sci. 2023. PMID: 37628722 Free PMC article. Review.
-
Choroid plexus transport: gene deletion studies.Fluids Barriers CNS. 2011 Nov 4;8(1):26. doi: 10.1186/2045-8118-8-26. Fluids Barriers CNS. 2011. PMID: 22053861 Free PMC article.
References
-
- Adachi, M., G. Reid, and J. D. Schuetz. 2002. Therapeutic and biological importance of getting nucleotides out of cells: a case for the ABC transporters, MRP4 and 5. Adv. Drug Deliv. Rev. 54:1333-1342. - PubMed
-
- Adachi, M., J. Sampath, L. B. Lan, D. Sun, P. Hargrove, R. Flatley, A. Tatum, M. Z. Edwards, M. Wezeman, L. Matherly, R. Drake, and J. Schuetz. 2002. Expression of MRP4 confers resistance to ganciclovir and compromises bystander cell killing. J. Biol. Chem. 277:38998-39004. - PubMed
-
- Ahmed, A. E., S. Jacob, J. P. Loh, S. K. Samra, M. Nokta, and R. B. Pollard. 1991. Comparative disposition and whole-body autoradiographic distribution of [2-14C]azidothymidine and [2-14C]thymidine in mice. J. Pharmacol. Exp. Ther. 257:479-486. - PubMed
-
- Ahmed, S. A., R. M. Gogal, Jr., and J. E. Walsh. 1994. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170:211-224. - PubMed
-
- Asaba, H., K. Hosoya, H. Takanaga, S. Ohtsuki, E. Tamura, T. Takizawa, and T. Terasaki. 2000. Blood-brain barrier is involved in the efflux transport of a neuroactive steroid, dehydroepiandrosterone sulfate, via organic anion transporting polypeptide 2. J. Neurochem. 75:1907-1916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
