Role of atypical protein kinase C in estradiol-triggered G1/S progression of MCF-7 cells
- PMID: 15314172
- PMCID: PMC506976
- DOI: 10.1128/MCB.24.17.7643-7653.2004
Role of atypical protein kinase C in estradiol-triggered G1/S progression of MCF-7 cells
Abstract
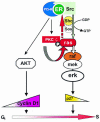
Expression of a dominant negative atypical protein kinase C (aPKC), PKCzeta, prevents nuclear translocation of extracellular regulated kinase 2 (ERK-2), p27 nuclear reduction, and DNA synthesis induced by estradiol in human mammary cancer-derived MCF-7 cells. aPKC action upstream of these events has been analyzed. In hormone-stimulated NIH 3T3 and Cos cells ectopically expressing human estrogen receptor alpha (hERalpha), aPKC is activated by phosphatidylinositol 3-kinase (PI 3-kinase) and, in turn, controls the Ras/MEK-1/ERK cascade. In MCF-7 and Cos cells stimulated by hormone, PI 3-kinase activates PKCzeta by Thr410 phosphorylation. Serine phosphorylation of PKCzeta is simultaneously induced. PKCzeta activation leads to recruitment of Ras to a multimolecular complex that also includes hERalpha, Src, PI 3-kinase, and aPKC. We propose that PKCzeta pushes Ras and the signaling complex close together in such a way that it facilitates the Src-dependent Ras activation. This activation is crucial for the interplay between estradiol-triggered signaling and cell cycle machinery.
Copyright 2004 American Society for Microbiology
Figures



Similar articles
-
Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells.EMBO J. 1996 Mar 15;15(6):1292-300. EMBO J. 1996. PMID: 8635462 Free PMC article.
-
Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence.Cancer Res. 2004 Oct 1;64(19):7156-68. doi: 10.1158/0008-5472.CAN-04-1121. Cancer Res. 2004. PMID: 15466214
-
Tyrosine phosphorylation of estradiol receptor by Src regulates its hormone-dependent nuclear export and cell cycle progression in breast cancer cells.Oncogene. 2012 Nov 15;31(46):4868-77. doi: 10.1038/onc.2011.642. Epub 2012 Jan 23. Oncogene. 2012. PMID: 22266855
-
Sex steroid hormones act as growth factors.J Steroid Biochem Mol Biol. 2002 Dec;83(1-5):31-5. doi: 10.1016/s0960-0760(02)00264-9. J Steroid Biochem Mol Biol. 2002. PMID: 12650699 Review.
-
Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions.J Biochem. 2003 Jan;133(1):1-7. doi: 10.1093/jb/mvg017. J Biochem. 2003. PMID: 12761192 Review.
Cited by
-
Carcinogenic effects of bisphenol A in breast and ovarian cancers.Oncol Lett. 2020 Dec;20(6):282. doi: 10.3892/ol.2020.12145. Epub 2020 Sep 23. Oncol Lett. 2020. PMID: 33014160 Free PMC article. Review.
-
DCPIB, a specific inhibitor of volume-regulated anion channels (VRACs), inhibits astrocyte proliferation and cell cycle progression via G1/S arrest.J Mol Neurosci. 2012 Feb;46(2):249-57. doi: 10.1007/s12031-011-9524-4. Epub 2011 May 11. J Mol Neurosci. 2012. PMID: 21559876
-
Roles of estrogen receptor alpha and beta in modulating urothelial cell proliferation.Endocr Relat Cancer. 2008 Mar;15(1):351-64. doi: 10.1677/erc.1.01255. Endocr Relat Cancer. 2008. PMID: 18310301 Free PMC article.
-
aPKC phosphorylates p27Xic1, providing a mechanistic link between apicobasal polarity and cell-cycle control.Dev Cell. 2014 Dec 8;31(5):559-71. doi: 10.1016/j.devcel.2014.10.023. Dev Cell. 2014. PMID: 25490266 Free PMC article.
-
Ashwagandha-Induced Programmed Cell Death in the Treatment of Breast Cancer.Curr Issues Mol Biol. 2024 Jul 18;46(7):7668-7685. doi: 10.3390/cimb46070454. Curr Issues Mol Biol. 2024. PMID: 39057095 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
