Single-chain estrogen receptors (ERs) reveal that the ERalpha/beta heterodimer emulates functions of the ERalpha dimer in genomic estrogen signaling pathways
- PMID: 15314175
- PMCID: PMC506997
- DOI: 10.1128/MCB.24.17.7681-7694.2004
Single-chain estrogen receptors (ERs) reveal that the ERalpha/beta heterodimer emulates functions of the ERalpha dimer in genomic estrogen signaling pathways
Abstract
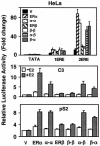
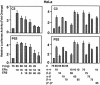
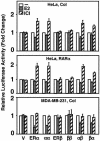
The effects of estrogens, particularly 17beta-estradiol (E2), are mediated by estrogen receptor alpha (ERalpha) and ERbeta. Upon binding to E2, ERs homo- and heterodimerize when coexpressed. The ER dimer then regulates the transcription of target genes through estrogen responsive element (ERE)-dependent and -independent pathways that constitute genomic estrogen signaling. Although ERalpha and ERbeta have similar ERE and E2 binding properties, they display different transregulatory capacities in both ERE-dependent and -independent signaling pathways. It is therefore likely that the heterodimerization provides novel functions to ERs by combining distinct properties of the contributing partners. The elucidation of the role of the ER heterodimer is critical for the understanding of physiology and pathophysiology of E2 signaling. However, differentially determining target gene responses during cosynthesis of ER subtypes is difficult, since dimers formed are a heterogeneous population of homo- and heterodimers. To circumvent the pivotal dimerization step in ER action and hence produce a homogeneous ER heterodimer population, we utilized a genetic fusion strategy. We joined the cDNAs of ERalpha and/or ERbeta to produce single-chain ERs to simulate the ER homo- and heterodimers. The fusion ERs interacted with ERE and E2 in a manner similar to that observed with the ER dimers. The homofusion receptors mimicked the functions of the parent ER dimers in the ERE-dependent and -independent pathways in transfected mammalian cells, whereas heterofusion receptors emulated the transregulatory properties of the ERalpha dimer. These results suggest that ERalpha is the functionally dominant partner in the ERalpha/beta heterodimer.
Copyright 2004 American Society for Microbiology
Figures







Similar articles
-
Binding of estrogen receptor beta to estrogen response element in situ is independent of estradiol and impaired by its amino terminus.Mol Endocrinol. 2005 Nov;19(11):2696-712. doi: 10.1210/me.2005-0120. Epub 2005 Jun 23. Mol Endocrinol. 2005. PMID: 15976006
-
Oestrogen receptors pathways to oestrogen responsive elements: the transactivation function-1 acts as the keystone of oestrogen receptor (ER)beta-mediated transcriptional repression of ERalpha.J Steroid Biochem Mol Biol. 2007 May;104(3-5):110-22. doi: 10.1016/j.jsbmb.2007.03.002. Epub 2007 Mar 12. J Steroid Biochem Mol Biol. 2007. PMID: 17478088
-
Estrogen receptor beta isoforms exhibit differences in ligand-activated transcriptional activity in an estrogen response element sequence-dependent manner.Endocrinology. 2004 Jan;145(1):149-60. doi: 10.1210/en.2003-1043. Epub 2003 Sep 18. Endocrinology. 2004. PMID: 14500565
-
Estrogen signaling: a subtle balance between ER alpha and ER beta.Mol Interv. 2003 Aug;3(5):281-92. doi: 10.1124/mi.3.5.281. Mol Interv. 2003. PMID: 14993442 Review.
-
Estrogen receptors: selective ligands, partners, and distinctive pharmacology.Recent Prog Horm Res. 2000;55:163-93; discussion 194-5. Recent Prog Horm Res. 2000. PMID: 11036937 Review.
Cited by
-
Negative regulation of estrogen signaling by ERβ and RIP140 in ovarian cancer cells.Mol Endocrinol. 2013 Sep;27(9):1429-41. doi: 10.1210/me.2012-1351. Epub 2013 Jul 24. Mol Endocrinol. 2013. PMID: 23885094 Free PMC article.
-
Estrogen signaling in the cardiovascular system.Nucl Recept Signal. 2006;4:e013. doi: 10.1621/nrs.04013. Epub 2006 Jul 7. Nucl Recept Signal. 2006. PMID: 16862219 Free PMC article.
-
Global analysis of estrogen receptor beta binding to breast cancer cell genome reveals an extensive interplay with estrogen receptor alpha for target gene regulation.BMC Genomics. 2011 Jan 14;12:36. doi: 10.1186/1471-2164-12-36. BMC Genomics. 2011. PMID: 21235772 Free PMC article.
-
WW domain-binding protein 2: an adaptor protein closely linked to the development of breast cancer.Mol Cancer. 2017 Jul 19;16(1):128. doi: 10.1186/s12943-017-0693-9. Mol Cancer. 2017. PMID: 28724435 Free PMC article. Review.
-
Somatic estrogen receptor α mutations that induce dimerization promote receptor activity and breast cancer proliferation.J Clin Invest. 2024 Jan 2;134(1):e163242. doi: 10.1172/JCI163242. J Clin Invest. 2024. PMID: 37883178 Free PMC article.
References
-
- Anzick, S. L., J. Kononen, R. L. Walker, D. O. Azorsa, M. M. Tanner, X. Y. Guan, G. Sauter, O. P. Kallioniemi, J. M. Trent, and P. S. Meltzer. 1997. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965-968. - PubMed
-
- Brzozowski, A. M., A. C. Pike, Z. Dauter, R. E. Hubbard, T. Bonn, O. Engstrom, L. Ohman, G. L. Greene, J. A. Gustafsson, and M. Carlquist. 1997. Molecular basis of agonism and antagonism in the estrogen receptor. Nature 389:753-758. - PubMed
-
- Cowley, S. M., S. Hoare, S. Mosselman, and M. G. Parker. 1997. Estrogen receptors α and β form heterodimers on DNA. J. Biol. Chem. 272:19858-19862. - PubMed
-
- Cowley, S. M., and M. G. Parker. 1999. A comparison of transcriptional activation by ER α and ERβ. J. Steroid Biochem. Mol. Biol. 69:165-175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
