Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling
- PMID: 15314219
- PMCID: PMC514658
- DOI: 10.1073/pnas.0404956101
Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling
Abstract
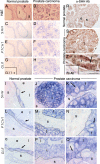
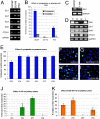
Prostate cancer is the most common solid tumor in men, and it shares with all cancers the hallmark of elevated, nonhomeostatic cell proliferation. Here we have tested the hypothesis that the SONIC HEDGEHOG (SHH)-GLI signaling pathway is implicated in prostate cancer. We report expression of SHH-GLI pathway components in adult human prostate cancer, often with enhanced levels in tumors versus normal prostatic epithelia. Blocking the pathway with cyclopamine or anti-SHH antibodies inhibits the proliferation of GLI1+/PSA+ primary prostate tumor cultures. Inversely, SHH can potentiate tumor cell proliferation, suggesting that autocrine signaling may often sustain tumor growth. In addition, pathway blockade in three metastatic prostate cancer cell lines with cyclopamine or through GLI1 RNA interference leads to inhibition of cell proliferation, suggesting cell-autonomous pathway activation at different levels and showing an essential role for GLI1 in human cells. Our data demonstrate the dependence of prostate cancer on SHH-GLI function and suggest a novel therapeutic approach.
Figures

References
-
- Ingham, P. & McMahon, A. (2001) Genes Dev. 15, 3059–3087. - PubMed
-
- Ruiz i Altaba, A., Sanchez, P. & Dahmane, N. (2002) Nat. Rev. Cancer 2, 361–372. - PubMed
-
- Pasca di Magliano, M. & Hebrok, M. (2003) Nat. Rev. Cancer 3, 903–911. - PubMed
-
- Hahn, H., Wicking, C., Zaphiropoulous, P. G., Gailani, M. R., Shanley, S., Chidambaram, A., Vorechovsky, I., Holmberg, E., Unden, A. B., Gillies, S., et al. (1996) Cell 85, 841–851. - PubMed
-
- Johnson, R. L., Rothman, A. L., Xie, J., Goodrich, L. V., Bare, J. W., Bonifas, J. M., Quinn, A. G., Myers, R. M., Cox, D. R., Epstein, E. H., Jr., & Scott, M. P. (1996) Science 272, 1668–1671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

