Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain
- PMID: 15314237
- PMCID: PMC515119
- DOI: 10.1073/pnas.0404915101
Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain
Abstract
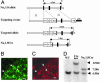
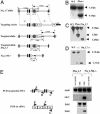
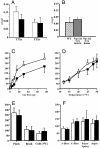
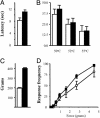
Nine voltage-gated sodium channels are expressed in complex patterns in mammalian nerve and muscle. Three channels, Na(v)1.7, Na(v)1.8, and Na(v)1.9, are expressed selectively in peripheral damage-sensing neurons. Because there are no selective blockers of these channels, we used gene ablation in mice to examine the function of Na(v)1.7 (PN1) in pain pathways. A global Na(v)1.7-null mutant was found to die shortly after birth. We therefore used the Cre-loxP system to generate nociceptor-specific knockouts. Na(v)1.8 is only expressed in peripheral, mainly nociceptive, sensory neurons. We knocked Cre recombinase into the Na(v)1.8 locus to generate heterozygous mice expressing Cre recombinase in Na(v)1.8-positive sensory neurons. Crossing these animals with mice where Na(v)1.7 exons 14 and 15 were flanked by loxP sites produced nociceptor-specific knockout mice that were viable and apparently normal. These animals showed increased mechanical and thermal pain thresholds. Remarkably, all inflammatory pain responses evoked by a range of stimuli, such as formalin, carrageenan, complete Freund's adjuvant, or nerve growth factor, were reduced or abolished. A congenital pain syndrome in humans recently has been mapped to the Na(v)1.7 gene, SCN9A. Dominant Na(v)1.7 mutations lead to edema, redness, warmth, and bilateral pain in human erythermalgia patients, confirming an important role for Na(v)1.7 in inflammatory pain. Nociceptor-specific gene ablation should prove useful in understanding the role of other broadly expressed genes in pain pathways.
Figures
References
-
- Catterall, W. A. (2000) Neuron 26, 13-25. - PubMed
-
- Wood, J. N., Akopian, A. N., Baker, M., Ding, Y., Geoghegan, F., Nassar, M., Malik-Hall, M., Okuse, K., Poon, L., Ravenall, S., et al. (2002) in Sodium Channels and Neuronal Hyperexcitability, Novartis Foundation Symposium (Wiley, West Sussex, U.K.), Vol. 241, pp. 159-68. - PubMed
-
- Akopian, A. N., Souslova, V., England, S., Okuse, K., Ogata, N., Ure, J., Smith, A., Kerr, B. J., McMahon, S. B., Boyce, S., et al. (1999) Nat. Neurosci. 2, 541-548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

