A focused and efficient genetic screening strategy in the mouse: identification of mutations that disrupt cortical development
- PMID: 15314648
- PMCID: PMC509294
- DOI: 10.1371/journal.pbio.0020219
A focused and efficient genetic screening strategy in the mouse: identification of mutations that disrupt cortical development
Abstract
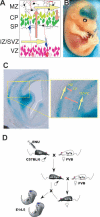
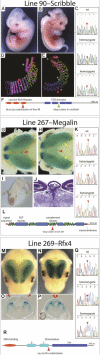
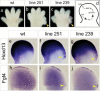
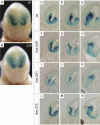
Although the mechanisms that regulate development of the cerebral cortex have begun to emerge, in large part through the analysis of mutant mice (Boncinelli et al. 2000; Molnar and Hannan 2000; Walsh and Goffinet 2000), many questions remain unanswered. To provide resources for further dissecting cortical development, we have carried out a focused screen for recessive mutations that disrupt cortical development. One aim of the screen was to identify mutants that disrupt the tangential migration of interneurons into the cortex. At the same time, we also screened for mutations that altered the growth or morphology of the cerebral cortex. We report here the identification of thirteen mutants with defects in aspects of cortical development ranging from the establishment of epithelial polarity to the invasion of thalamocortical axons. Among the collection are three novel alleles of genes for which mutant alleles had already been used to explore forebrain development, and four mutants with defects in interneuron migration. The mutants that we describe here will aid in deciphering the molecules and mechanisms that regulate cortical development. Our results also highlight the utility of focused screens in the mouse, in addition to the large-scale and broadly targeted screens that are being carried out at mutagenesis centers.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures


Similar articles
-
A forward genetic screen with a thalamocortical axon reporter mouse yields novel neurodevelopment mutants and a distinct emx2 mutant phenotype.Neural Dev. 2011 Jan 7;6:3. doi: 10.1186/1749-8104-6-3. Neural Dev. 2011. PMID: 21214893 Free PMC article.
-
A forward genetic screen in mice identifies mutants with abnormal cortical patterning.Cereb Cortex. 2015 Jan;25(1):167-79. doi: 10.1093/cercor/bht209. Epub 2013 Aug 22. Cereb Cortex. 2015. PMID: 23968836 Free PMC article.
-
Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain.Development. 2006 Jun;133(11):2243-52. doi: 10.1242/dev.02379. Development. 2006. PMID: 16690755
-
Differential gene expression in migrating cortical interneurons during mouse forebrain development.J Comp Neurol. 2010 Apr 15;518(8):1232-48. doi: 10.1002/cne.22271. J Comp Neurol. 2010. PMID: 20151419
-
N-ethyl-N-nitrosourea mutagenesis: boarding the mouse mutant express.Microbiol Mol Biol Rev. 2005 Sep;69(3):426-39. doi: 10.1128/MMBR.69.3.426-439.2005. Microbiol Mol Biol Rev. 2005. PMID: 16148305 Free PMC article. Review.
Cited by
-
Meningeal defects alter the tangential migration of cortical interneurons in Foxc1hith/hith mice.Neural Dev. 2012 Jan 17;7:2. doi: 10.1186/1749-8104-7-2. Neural Dev. 2012. PMID: 22248045 Free PMC article.
-
Using microarrays to facilitate positional cloning: identification of tomosyn as an inhibitor of neurosecretion.PLoS Genet. 2005 Jul;1(1):6-16. doi: 10.1371/journal.pgen.0010002. Epub 2005 Jul 25. PLoS Genet. 2005. PMID: 16103915 Free PMC article.
-
Epidermal wound repair is regulated by the planar cell polarity signaling pathway.Dev Cell. 2010 Jul 20;19(1):138-47. doi: 10.1016/j.devcel.2010.06.008. Dev Cell. 2010. PMID: 20643356 Free PMC article.
-
The ciliogenic transcription factor RFX3 regulates early midline distribution of guidepost neurons required for corpus callosum development.PLoS Genet. 2012;8(3):e1002606. doi: 10.1371/journal.pgen.1002606. Epub 2012 Mar 29. PLoS Genet. 2012. PMID: 22479201 Free PMC article.
-
A mutation in mouse Pak1ip1 causes orofacial clefting while human PAK1IP1 maps to 6p24 translocation breaking points associated with orofacial clefting.PLoS One. 2013 Jul 25;8(7):e69333. doi: 10.1371/journal.pone.0069333. Print 2013. PLoS One. 2013. PMID: 23935987 Free PMC article.
References
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: Dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
-
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
-
- Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. - PubMed
-
- Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

