Gcn4p and novel upstream activating sequences regulate targets of the unfolded protein response
- PMID: 15314660
- PMCID: PMC509306
- DOI: 10.1371/journal.pbio.0020246
Gcn4p and novel upstream activating sequences regulate targets of the unfolded protein response
Abstract
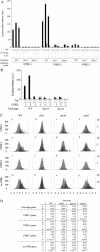
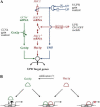
Eukaryotic cells respond to accumulation of unfolded proteins in the endoplasmic reticulum (ER) by activating the unfolded protein response (UPR), a signal transduction pathway that communicates between the ER and the nucleus. In yeast, a large set of UPR target genes has been experimentally determined, but the previously characterized unfolded protein response element (UPRE), an upstream activating sequence (UAS) found in the promoter of the UPR target gene KAR2, cannot account for the transcriptional regulation of most genes in this set. To address this puzzle, we analyzed the promoters of UPR target genes computationally, identifying as candidate UASs short sequences that are statistically overrepresented. We tested the most promising of these candidate UASs for biological activity, and identified two novel UPREs, which are necessary and sufficient for UPR activation of promoters. A genetic screen for activators of the novel motifs revealed that the transcription factor Gcn4p plays an essential and previously unrecognized role in the UPR: Gcn4p and its activator Gcn2p are required for induction of a majority of UPR target genes during ER stress. Both Hac1p and Gcn4p bind target gene promoters to stimulate transcriptional induction. Regulation of Gcn4p levels in response to changing physiological conditions may function as an additional means to modulate the UPR. The discovery of a role for Gcn4p in the yeast UPR reveals an additional level of complexity and demonstrates a surprising conservation of the signaling circuit between yeast and metazoan cells.
Conflict of interest statement
The authors declare that no conflicts of interest exist.
Figures






References
-
- Albrecht G, Mosch HU, Hoffmann B, Reusser U, Braus GH. Monitoring the Gcn4 protein-mediated response in the yeast Saccharomyces cerevisiae . J Biol Chem. 1998;273:12696–12702. - PubMed
-
- Ausubel FM, editor . New York: Greene/Wiley Interscience; 1988. Current protocols in molecular biology, Volumes 1–4.
-
- Ben-Dor A, Shamir R, Yakhini Z. Clustering gene expression patterns. J Comput Biol. 1999;6:281–297. - PubMed
-
- Bussemaker HJ, Li H, Siggia ED. Regulatory element detection using a probabilistic segmentation model. Proc Int Conf Intell Syst Mol Biol. 2000b;8:67–74. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

