Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease
- PMID: 15314690
- PMCID: PMC503769
- DOI: 10.1172/JCI21109
Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease
Abstract
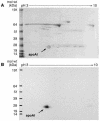
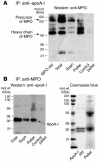
In recent studies we demonstrated that systemic levels of protein-bound nitrotyrosine (NO(2)Tyr) and myeloperoxidase (MPO), a protein that catalyzes generation of nitrating oxidants, serve as independent predictors of atherosclerotic risk, burden, and incident cardiac events. We now show both that apolipoprotein A-I (apoA-I), the primary protein constituent of HDL, is a selective target for MPO-catalyzed nitration and chlorination in vivo and that MPO-catalyzed oxidation of HDL and apoA-I results in selective inhibition in ABCA1-dependent cholesterol efflux from macrophages. Dramatic selective enrichment in NO(2)Tyr and chlorotyrosine (ClTyr) content within apoA-I recovered from serum and human atherosclerotic lesions is noted, and analysis of serum from sequential subjects demonstrates that the NO(2)Tyr and ClTyr contents of apoA-I are markedly higher in individuals with cardiovascular disease (CVD). Analysis of circulating HDL further reveals that higher NO(2)Tyr and ClTyr contents of the lipoprotein are each significantly associated with diminished ABCA1-dependent cholesterol efflux capacity of the lipoprotein. MPO as a likely mechanism for oxidative modification of apoA-I in vivo is apparently facilitated by MPO binding to apoA-I, as revealed by cross-immunoprecipitation studies in plasma, recovery of MPO within HDL-like particles isolated from human atheroma, and identification of a probable contact site between the apoA-I moiety of HDL and MPO. To our knowledge, the present results provide the first direct evidence for apoA-I as a selective target for MPO-catalyzed oxidative modification in human atheroma. They also suggest a potential mechanism for MPO-dependent generation of a proatherogenic dysfunctional form of HDL in vivo.
Figures





References
-
- Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic. Biol. Med. 2000;28:1815–1826. - PubMed
-
- Navab M, et al. Oxidized lipids as mediators of coronary heart disease. Curr. Opin. Lipidol. 2002;13:363–372. - PubMed
-
- Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic. Biol. Med. 2000;28:1717–1725. - PubMed
-
- Heinecke JW. Oxidative stress: new approaches to diagnosis and prognosis in atherosclerosis. Am. J. Cardiol. 2003;91:12A–16A. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL066082/HL/NHLBI NIH HHS/United States
- P50 HL 70128/HL/NHLBI NIH HHS/United States
- M01 RR 108390/RR/NCRR NIH HHS/United States
- R01 HL070621/HL/NHLBI NIH HHS/United States
- P01 HL076491/HL/NHLBI NIH HHS/United States
- RR 16794/RR/NCRR NIH HHS/United States
- M01 RR018390/RR/NCRR NIH HHS/United States
- P01 HL 076491/HL/NHLBI NIH HHS/United States
- HL 62526/HL/NHLBI NIH HHS/United States
- HL 70621/HL/NHLBI NIH HHS/United States
- P50 HL070128/HL/NHLBI NIH HHS/United States
- 1S10 RR 15794/RR/NCRR NIH HHS/United States
- R01 HL077692/HL/NHLBI NIH HHS/United States
- HL 66082/HL/NHLBI NIH HHS/United States
- HL 077692/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

