Poor immunogenicity of a self/tumor antigen derives from peptide-MHC-I instability and is independent of tolerance
- PMID: 15314692
- PMCID: PMC503773
- DOI: 10.1172/JCI21695
Poor immunogenicity of a self/tumor antigen derives from peptide-MHC-I instability and is independent of tolerance
Abstract
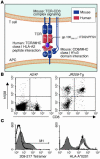
Understanding the mechanisms underlying the poor immunogenicity of human self/tumor antigens is challenging because of experimental limitations in humans. Here, we developed a human-mouse chimeric model that allows us to investigate the roles of the frequency and self-reactivity of antigen-specific T cells in determination of the immunogenicity of an epitope (amino acids 209-217) derived from a human melanoma antigen, gp100. In these transgenic mice, CD8+ T cells express the variable regions of a human T cell receptor (hTCR) specific for an HLA-A*0201-restricted gp100(209-217). Immunization of hTCR-transgenic mice with gp100(209-217) peptide elicited minimal T cell responses, even in mice in which the epitope was knocked out. Conversely, a modified epitope, gp100(209-217(2M)), was significantly more immunogenic. Both biological and physical assays revealed a fast rate of dissociation of the native peptide from the HLA-A*0201 molecule and a considerably slower rate of dissociation of the modified peptide. In vivo, the time allowed for dissociation of peptide-MHC complexes on APCs prior to their exposure to T cells significantly affected the induction of immune responses. These findings indicate that the poor immunogenicity of some self/tumor antigens is due to the instability of the peptide-MHC complex rather than to the continual deletion or tolerization of self-reactive T cells.
Figures
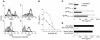

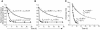
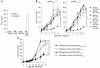
Comment in
-
Immune recognition of self in immunity against cancer.J Clin Invest. 2004 Aug;114(4):468-71. doi: 10.1172/JCI22685. J Clin Invest. 2004. PMID: 15314682 Free PMC article. Review.
References
-
- Van den Eynde, B., and van der Bruggen, P. 2003. Peptide database of T-cell defined tumor antigens. Cancer Immunity. [serial online]. http://www.cancerimmunity.org/peptidedatabase/Tcellepitopes.htm#text.
-
- Kawakami Y, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J. Immunol. 1995;154:3961–3968. - PubMed
-
- Parkhurst MR, et al. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J. Immunol. 1996;157:2539–2548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

