Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes
- PMID: 15314696
- PMCID: PMC503771
- DOI: 10.1172/JCI21307
Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes
Abstract
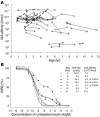
Children at risk for type 1 diabetes can develop early insulin autoantibodies (IAAs). Many, but not all, of these children subsequently develop multiple islet autoantibodies and diabetes. To determine whether disease progression is reflected by autoantibody maturity, IAA affinity was measured by competitive radiobinding assay in first and subsequent IAA-positive samples from children followed from birth in the BABYDIAB cohort. IAA affinity in first positive samples ranged from less than 10(6) l/mol to more than 10(11) l/mol. High affinity was associated with HLA DRB1*04, young age of IAA appearance, and subsequent progression to multiple islet autoantibodies or type 1 diabetes. IAA affinity in multiple antibody-positive children was on average 100-fold higher than in children who remained single IAA positive or became autoantibody negative. All high-affinity IAAs required conservation of human insulin A chain residues 8-13 and were reactive with proinsulin. In contrast, most lower-affinity IAAs were dependent on COOH-terminal B chain residues and did not bind proinsulin. These data are consistent with the concept that type 1 diabetes is associated with sustained early exposure to (pro)insulin in the context of HLA DR4 and show that high-affinity proinsulin-reactive IAAs identify children with the highest diabetes risk.
Figures



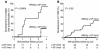


References
-
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. - PubMed
-
- Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48:460–468. - PubMed
-
- Kimpimaki T, et al. Natural history of beta-cell autoimmunity in young children with increased genetic susceptibility to type 1 diabetes recruited from the general population. J. Clin. Endocrinol. Metab. 2002;87:4572–4579. - PubMed
-
- Colman PG, et al. Islet autoimmunity in infants with a Type I diabetic relative is common but is frequently restricted to one autoantibody. Diabetologia. 2000;43:203–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

