A gain-of-function mutation of Fgfr2c demonstrates the roles of this receptor variant in osteogenesis
- PMID: 15316116
- PMCID: PMC515096
- DOI: 10.1073/pnas.0405031101
A gain-of-function mutation of Fgfr2c demonstrates the roles of this receptor variant in osteogenesis
Abstract
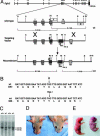
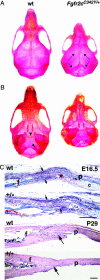
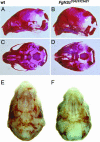
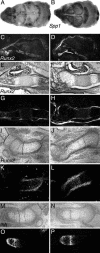
The b and c variants of fibroblast growth factor receptor 2 (FGFR2) differ in sequence, binding specificity, and localization. Fgfr2b, expressed in epithelia, is required for limb outgrowth and branching morphogenesis, whereas the mesenchymal Fgfr2c variant is required by the osteocyte lineage for normal skeletogenesis. Gain-of-function mutations in human FGFR2c are associated with craniosynostosis syndromes. To confirm and extend this evidence, we introduced a Cys342Tyr replacement into Fgfr2c to create a gain-of-function mutation equivalent to a mutation in human Crouzon and Pfeiffer syndromes. Fgfr2c(C342Y/)(+) heterozygote mice are viable and fertile with shortened face, protruding eyes, premature fusion of cranial sutures, and enhanced Spp1 expression in the calvaria. Homozygous mutants display multiple joint fusions, cleft palate, and trachea and lung defects, and die shortly after birth. They show enhanced Cbfa1/Runx2 expression without significant change in chondrocyte-specific Ihh, PTHrP, Sox9, Col2a, or Col10a gene expression. Histomorphometric analysis and bone marrow stromal cell culture showed a significant increase of osteoblast progenitors with no change in osteoclastogenic cells. Chondrocyte proliferation was decreased in the skull base at embryonic day 14.5 but not later. These results suggest that long-term aspects of the mutant phenotype, including craniosynostosis, are related to the Fgfr2c regulation of the osteoblast lineage. The effect on early chondrocyte proliferation but not gene expression suggests cooperation of Fgfr2c with Fgfr3 in the formation of the cartilage model for endochondral bone.
Figures

Similar articles
-
Overexpression of Fgfr2c causes craniofacial bone hypoplasia and ameliorates craniosynostosis in the Crouzon mouse.Dis Model Mech. 2018 Nov 9;11(11):dmm035311. doi: 10.1242/dmm.035311. Dis Model Mech. 2018. PMID: 30266836 Free PMC article.
-
Further analysis of the Crouzon mouse: effects of the FGFR2(C342Y) mutation are cranial bone-dependent.Calcif Tissue Int. 2013 May;92(5):451-66. doi: 10.1007/s00223-013-9701-2. Epub 2013 Jan 29. Calcif Tissue Int. 2013. PMID: 23358860 Free PMC article.
-
The IIIc alternative of Fgfr2 is a positive regulator of bone formation.Development. 2002 Aug;129(16):3783-93. doi: 10.1242/dev.129.16.3783. Development. 2002. PMID: 12135917
-
Fibroblast growth factor receptor signaling crosstalk in skeletogenesis.Sci Signal. 2010 Nov 2;3(146):re9. doi: 10.1126/scisignal.3146re9. Sci Signal. 2010. PMID: 21045207 Review.
-
Craniosynostosis and related limb anomalies.Novartis Found Symp. 2001;232:122-33; discussion 133-43. doi: 10.1002/0470846658.ch9. Novartis Found Symp. 2001. PMID: 11277076 Review.
Cited by
-
Bent bone dysplasia-FGFR2 type, a distinct skeletal disorder, has deficient canonical FGF signaling.Am J Hum Genet. 2012 Mar 9;90(3):550-7. doi: 10.1016/j.ajhg.2012.02.005. Epub 2012 Mar 1. Am J Hum Genet. 2012. PMID: 22387015 Free PMC article.
-
Mesenchymal fibroblast growth factor receptor signaling regulates palatal shelf elevation during secondary palate formation.Dev Dyn. 2015 Nov;244(11):1427-38. doi: 10.1002/dvdy.24319. Epub 2015 Aug 24. Dev Dyn. 2015. PMID: 26250517 Free PMC article.
-
Wnt/β-catenin signaling and Msx1 promote outgrowth of the maxillary prominences.Front Physiol. 2012 Sep 21;3:375. doi: 10.3389/fphys.2012.00375. eCollection 2012. Front Physiol. 2012. PMID: 23055979 Free PMC article.
-
The CMS19 disease model specifies a pivotal role for collagen XIII in bone homeostasis.Sci Rep. 2022 Apr 7;12(1):5866. doi: 10.1038/s41598-022-09653-4. Sci Rep. 2022. PMID: 35393492 Free PMC article.
-
Bent bone dysplasia syndrome reveals nucleolar activity for FGFR2 in ribosomal DNA transcription.Hum Mol Genet. 2014 Nov 1;23(21):5659-71. doi: 10.1093/hmg/ddu282. Epub 2014 Jun 6. Hum Mol Genet. 2014. PMID: 24908667 Free PMC article.
References
-
- Ornitz, D. M., Xu, J., Colvin, J. S., McEwen, D. G., MacArthur, C. A., Coulier, F., Gao, G. & Goldfarb, M. (1996) J. Biol. Chem. 271, 15292–15297. - PubMed
-
- Orr-Urtreger, A., Bedford, M. T., Burakova, T., Arman, E., Zimmer, Y., Yayon, A., Givol, D. & Lonai, P. (1993) Dev. Biol. 158, 475–486. - PubMed
-
- De Moerlooze, L., Spencer-Dene, B., Revest, J., Hajihosseini, M., Rosewell, I. & Dickson, C. (2000) Development (Cambridge, U.K.) 127, 483–492. - PubMed
-
- Eswarakumar, V. P., Monsonego-Ornan, E., M. Pines, M., Antonopoulou, I., Morriss-Kay, G. M. & Lonai, P. (2002) Development (Cambridge, U.K.) 129, 3783–3793. - PubMed
-
- Gorivodsky, M. & Lonai, P. (2003) Development (Cambridge, U.K.) 130, 5471–5479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

