Oxytocin in cardiac ontogeny
- PMID: 15316117
- PMCID: PMC516519
- DOI: 10.1073/pnas.0405324101
Oxytocin in cardiac ontogeny
Abstract
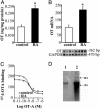
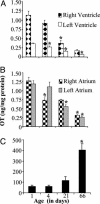
Previous studies demonstrated the presence of oxytocin (OT) and oxytocin receptors (OTRs) in the heart. The present work provides results supporting a potential role of OT in cardiomyogenesis. Here, we show a maximal OT and OTR protein level in the developing rat heart at day 21 of gestation and postnatal days 1-4, when cardiac myocytes are at a stage of intense hyperplasia. Between postnatal days 1 and 66, OT decreased linearly in all heart chambers (4.1- to 6.6-fold). Correspondingly, immunocytochemistry demonstrated that OTRs, which were eminent in postnatal cardiomyocytes, declined with age to low levels in adults. Interestingly, in coronary vasculature, OTRs developed in endothelial cells at postnatal days 12 and 22 and achieved a plateau in adult rats. These findings suggest that OT can be involved in developmental formation of the coronary vessels. In vivo, the OT/OTR system in the fetal heart was sensitive to the actions of retinoic acid (RA), recognized as a major cardiac morphogen. RA treatment produced a significant increase (2- to 3-fold) both in the OT concentration and in the OT mRNA levels. Ex vivo, an OT antagonist inhibited RA-mediated cardiomyocyte differentiation of P19 embryonic stem cells. The decline of cardiac OT expression from infancy to adulthood of the rat and changes in cell types expressing OTR indicate a dynamic regulation of the OT system in the heart rather than constitutive expression. The results support the hypothesis that RA induces cardiomyogenesis by activation of the cardiac OT system.
Copyright 2004 The National Academy of Sciencs of the USA
Figures




Similar articles
-
Oxytocin induces differentiation of P19 embryonic stem cells to cardiomyocytes.Proc Natl Acad Sci U S A. 2002 Jul 9;99(14):9550-5. doi: 10.1073/pnas.152302499. Epub 2002 Jul 1. Proc Natl Acad Sci U S A. 2002. PMID: 12093924 Free PMC article.
-
Oxytocin-Gly-Lys-Arg stimulates cardiomyogenesis by targeting cardiac side population cells.J Endocrinol. 2014 Jan 30;220(3):277-89. doi: 10.1530/JOE-13-0305. Print 2014 Mar. J Endocrinol. 2014. PMID: 24403294
-
Expression of heart oxytocin receptor and its mRNA in two rat strains with different activity of HPA axis.Neuro Endocrinol Lett. 2011;32(6):805-10. Neuro Endocrinol Lett. 2011. PMID: 22286791
-
Oxytocin and cardioprotection in diabetes and obesity.BMC Endocr Disord. 2016 Jun 7;16(1):34. doi: 10.1186/s12902-016-0110-1. BMC Endocr Disord. 2016. PMID: 27268060 Free PMC article. Review.
-
Oxytocin revisited: its role in cardiovascular regulation.J Neuroendocrinol. 2012 Apr;24(4):599-608. doi: 10.1111/j.1365-2826.2011.02235.x. J Neuroendocrinol. 2012. PMID: 21981277 Review.
Cited by
-
The Heart as a Target of Vasopressin and Other Cardiovascular Peptides in Health and Cardiovascular Diseases.Int J Mol Sci. 2022 Nov 20;23(22):14414. doi: 10.3390/ijms232214414. Int J Mol Sci. 2022. PMID: 36430892 Free PMC article. Review.
-
The Role of Oxytocin in Cardiovascular Protection.Front Psychol. 2020 Aug 25;11:2139. doi: 10.3389/fpsyg.2020.02139. eCollection 2020. Front Psychol. 2020. PMID: 32982875 Free PMC article. Review.
-
A journey from speech to dance through the field of oxytocin.Compr Psychoneuroendocrinol. 2023 Jul 31;16:100193. doi: 10.1016/j.cpnec.2023.100193. eCollection 2023 Nov. Compr Psychoneuroendocrinol. 2023. PMID: 38108035 Free PMC article. Review.
-
Vasopressin & Oxytocin in Control of the Cardiovascular System: An Updated Review.Curr Neuropharmacol. 2020;18(1):14-33. doi: 10.2174/1570159X17666190717150501. Curr Neuropharmacol. 2020. PMID: 31544693 Free PMC article. Review.
-
Aging and age-related diseases with a focus on therapeutic potentials of young blood/plasma.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jan;397(1):1-13. doi: 10.1007/s00210-023-02657-5. Epub 2023 Aug 8. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37552316 Review.
References
-
- Gimpl, G. & Fahrenholz, F. (2001) Physiol. Rev. 81, 629-683. - PubMed
-
- McCann, S. M., Antunes-Rodrigues, J., Jankowski, M. & Gutkowska, J. (2002) Prog. Brain Res. 130, 309-328. - PubMed
-
- Antunes-Rodrigues, J., de Castro., M, Elias, L. L., Valenca, M. M. & McCann, S. M. (2004) Physiol. Rev. 8, 169-208. - PubMed
-
- Petersson, M., Alster P., Lundberg, T. & Uvnas-Moberg, K. (1996) Physiol. Behav. 60, 1311-1315. - PubMed
-
- Favaretto, A. L., Ballejo, G. O., Albuquerque-Araujo, W. I., Gutkowska, J., Antunes-Rodrigues, J. & McCann, S. M. (1997) Peptides 18, 1377-1381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

