Decreased neointimal formation in Nox2-deficient mice reveals a direct role for NADPH oxidase in the response to arterial injury
- PMID: 15316118
- PMCID: PMC516510
- DOI: 10.1073/pnas.0405389101
Decreased neointimal formation in Nox2-deficient mice reveals a direct role for NADPH oxidase in the response to arterial injury
Abstract
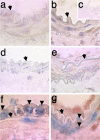
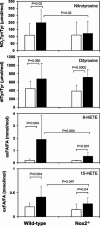
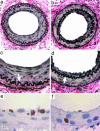
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced, in part, from NADPH oxidase in response to host invasion and tissue injury. Defects in NADPH oxidase impair host defense; however, the role of ROS and RNS in the response to tissue injury is not known. We addressed this issue by subjecting leukocyte oxidase (Nox2)-deficient (Nox2-/-) mice to arterial injury. Femoral artery injury was associated with increased Nox2 expression, ROS/RNS production, and oxidative protein and lipid modification in wild-type mice. In Nox2-/- mice, RNS-mediated protein oxidation, as monitored by protein nitrotyrosine content, was significantly diminished. This was accompanied by reduced neointimal proliferation, as monitored by intimal thickness and intimal/medial ratio, in Nox2-/- compared to wild-type mice. In addition, Nox2 deficiency led to reduced cellular proliferation and leukocyte accumulation. These data indicate that Nox2-mediated oxidant production has a requisite role in the response to tissue injury.
Copyright 2004 The National Academy of Sciencs of the USA
Figures

Similar articles
-
Nox2-containing NADPH oxidase deficiency confers protection from hindlimb ischemia in conditions of increased oxidative stress.Arterioscler Thromb Vasc Biol. 2009 Oct;29(10):1522-8. doi: 10.1161/ATVBAHA.109.191437. Epub 2009 Jul 2. Arterioscler Thromb Vasc Biol. 2009. PMID: 19574557
-
Role of NADPH oxidase isoforms NOX1, NOX2 and NOX4 in myocardial ischemia/reperfusion injury.J Mol Cell Cardiol. 2013 Nov;64:99-107. doi: 10.1016/j.yjmcc.2013.09.007. Epub 2013 Sep 16. J Mol Cell Cardiol. 2013. PMID: 24051369
-
Arterial hypertension in a murine model of sleep apnea: role of NADPH oxidase 2.J Hypertens. 2014 Feb;32(2):300-5. doi: 10.1097/HJH.0000000000000016. J Hypertens. 2014. PMID: 24270180
-
Phagocyte NADPH oxidase and specific immunity.Clin Sci (Lond). 2015 May 1;128(10):635-48. doi: 10.1042/CS20140635. Clin Sci (Lond). 2015. PMID: 25760962 Review.
-
NOX2 complex-derived ROS as immune regulators.Antioxid Redox Signal. 2011 Oct 15;15(8):2197-208. doi: 10.1089/ars.2010.3635. Epub 2011 Apr 11. Antioxid Redox Signal. 2011. PMID: 20919938 Review.
Cited by
-
The Role of Oxidants in Percutaneous Coronary Intervention-Induced Endothelial Dysfunction: Can We Harness Redox Signaling to Improve Clinical Outcomes?Antioxid Redox Signal. 2023 May;38(13-15):1022-1040. doi: 10.1089/ars.2022.0204. Epub 2023 Mar 7. Antioxid Redox Signal. 2023. PMID: 36641638 Free PMC article. Review.
-
C/EBP transcription factors regulate NADPH oxidase in human aortic smooth muscle cells.J Cell Mol Med. 2014 Jul;18(7):1467-77. doi: 10.1111/jcmm.12289. Epub 2014 May 6. J Cell Mol Med. 2014. PMID: 24797079 Free PMC article.
-
p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases.Exp Mol Med. 2009 Apr 30;41(4):217-25. doi: 10.3858/emm.2009.41.4.058. Exp Mol Med. 2009. PMID: 19372727 Free PMC article. Review.
-
Smooth muscle specific overexpression of p22phox potentiates carotid artery wall thickening in response to injury.Oxid Med Cell Longev. 2015;2015:305686. doi: 10.1155/2015/305686. Epub 2015 Apr 5. Oxid Med Cell Longev. 2015. PMID: 25945151 Free PMC article.
-
Evolution of NADPH Oxidase Inhibitors: Selectivity and Mechanisms for Target Engagement.Antioxid Redox Signal. 2015 Aug 10;23(5):406-27. doi: 10.1089/ars.2013.5814. Epub 2014 Feb 26. Antioxid Redox Signal. 2015. PMID: 24383718 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- HL73852/HL/NHLBI NIH HHS/United States
- R37 HL057506/HL/NHLBI NIH HHS/United States
- HL077692/HL/NHLBI NIH HHS/United States
- R01 HL070621/HL/NHLBI NIH HHS/United States
- HL076491/HL/NHLBI NIH HHS/United States
- HL70621/HL/NHLBI NIH HHS/United States
- P01 HL076491/HL/NHLBI NIH HHS/United States
- R01 HL073852/HL/NHLBI NIH HHS/United States
- M01 RR018390/RR/NCRR NIH HHS/United States
- HL57506/HL/NHLBI NIH HHS/United States
- R01 HL057506/HL/NHLBI NIH HHS/United States
- DK55875/DK/NIDDK NIH HHS/United States
- R01 HL077692/HL/NHLBI NIH HHS/United States
- R01 DK055875/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

