An intravitreal biodegradable sustained release naproxen and 5-fluorouracil system for the treatment of experimental post-traumatic proliferative vitreoretinopathy
- PMID: 15317716
- PMCID: PMC1772295
- DOI: 10.1136/bjo.2003.039917
An intravitreal biodegradable sustained release naproxen and 5-fluorouracil system for the treatment of experimental post-traumatic proliferative vitreoretinopathy
Abstract
Background/aims: To determine the potential of an intravitreal sustained release naproxen and 5-fluorouracil (NA/5-FU) codrug for the treatment of experimental proliferative vitreoretinopathy (PVR) in a model for trauma associated tractional retinal detachment (TRD).
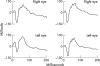
Methods: Sustained release pellets were prepared by covalently linking naproxen to 5-fluorouracil. Drug release was tested in vitro and toxic effects were evaluated by electroretinography and light microscopy. Traumatic PVR was induced in pigmented rabbits by performing a scleral laceration, followed by repair and intravitreal injection of 0.4 ml of autologous blood. Thirty six eyes were treated with a sustained release implant containing 1.5 mg NA/5-FU as a codrug and 36 control eyes were submitted to surgery alone. Eyes were evaluated for TRD by serial indirect ophthalmoscope examination at different time points followed by postmortem fundus evaluation of the enucleated eye
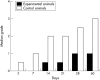
Results: The NA/5-FU pellets were found to provide linear release of 5-FU and naproxen over the 30 day duration of the in vitro release test. Both the severity of PVR grade and the percentage of eyes with moderate or worse tractional detachment were significantly lower in eyes treated with the codrug pellet. There were no drug related toxic effects evident on histopathological or electroretinograph examination of eyes containing the NA/5-FU pellet.
Conclusions: The results suggest that this NA/5-FU codrug device effectively inhibits the progression of PVR in a rabbit trauma model that closely resembles PVR in humans. Additional studies to add knowledge to these initial findings and to clarify the potential of the codrug device for the treatment of human PVR are warranted.
Figures



Similar articles
-
An intravitreal sustained-release triamcinolone and 5-fluorouracil codrug in the treatment of experimental proliferative vitreoretinopathy.Arch Ophthalmol. 1998 Jan;116(1):69-77. doi: 10.1001/archopht.116.1.69. Arch Ophthalmol. 1998. PMID: 9445210
-
Intravitreal sustained release corticosteroid-5-fluoruracil conjugate in the treatment of experimental proliferative vitreoretinopathy.Invest Ophthalmol Vis Sci. 1996 Oct;37(11):2318-25. Invest Ophthalmol Vis Sci. 1996. PMID: 8843916
-
Liposome-encapsulated 5-fluorouracil in the treatment of proliferative vitreoretinopathy.Ophthalmic Surg. 1988 Apr;19(4):252-6. Ophthalmic Surg. 1988. PMID: 3362494
-
[Proliferative vitreoretinopathy: prophylactic treatment].J Fr Ophtalmol. 2014 Nov;37(9):737-43. doi: 10.1016/j.jfo.2014.04.003. Epub 2014 Jul 8. J Fr Ophtalmol. 2014. PMID: 25012973 Review. French.
-
Clinical management of proliferative vitreoretinopathy: an update.Retina. 2015 Feb;35(2):165-75. doi: 10.1097/IAE.0000000000000447. Retina. 2015. PMID: 25602631 Review.
Cited by
-
The clinical applications of fluorouracil in ophthalmic practice.Drugs. 2007;67(2):237-55. doi: 10.2165/00003495-200767020-00005. Drugs. 2007. PMID: 17284086 Review.
-
Biodegradable intraocular therapies for retinal disorders: progress to date.Drugs Aging. 2010 Feb 1;27(2):117-34. doi: 10.2165/11530970-000000000-00000. Drugs Aging. 2010. PMID: 20104938 Review.
-
Effect of different fixative solutions on eyes with experimental proliferative vitreoretinopathy.Int J Exp Pathol. 2015 Apr;96(2):103-10. doi: 10.1111/iep.12119. Epub 2015 Feb 9. Int J Exp Pathol. 2015. PMID: 25670226 Free PMC article.
-
[Pharmacological approach to treatment of proliferative vitreoretinopathy].Ophthalmologe. 2013 Oct;110(10):948-59. doi: 10.1007/s00347-013-2832-z. Ophthalmologe. 2013. PMID: 24046169 Review. German.
-
Sustained dasatinib treatment prevents early fibrotic changes following ocular trauma.Graefes Arch Clin Exp Ophthalmol. 2021 May;259(5):1103-1111. doi: 10.1007/s00417-020-05037-4. Epub 2021 Jan 8. Graefes Arch Clin Exp Ophthalmol. 2021. PMID: 33417094 Free PMC article.
References
-
- Parver LM. Eye trauma: the neglected disorder. Arch Ophthalmol 1986;104:1452–3. - PubMed
-
- Cardillo JA, Stout T, LaBree L, et al. Post-traumatic proliferative vitreoretinopathy. The epidemiologic profile, onset, risk factors, and visual outcome. Ophthalmology 1997;104:1166–73. - PubMed
-
- Eagling EM. Perforating injuries involving the posterior segment. Trans Ophthalmol Soc UK 1975;95:335–9. - PubMed
-
- Cox MS, Freeman HM. Retinal detachment due to ocular penetration I. Clinical characteristics and surgical results. Arch Ophthalmol 1978;96:1354–61. - PubMed
-
- Ryan SJ, Allen AW. Pars plana vitrectomy in ocular trauma. Am J Ophthalmol 1979;88:483–91. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
