Functional characterization of an aminotransferase required for pyoverdine siderophore biosynthesis in Pseudomonas aeruginosa PAO1
- PMID: 15317763
- PMCID: PMC516838
- DOI: 10.1128/JB.186.17.5596-5602.2004
Functional characterization of an aminotransferase required for pyoverdine siderophore biosynthesis in Pseudomonas aeruginosa PAO1
Abstract
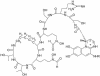
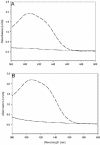
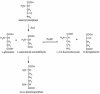
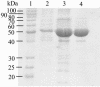
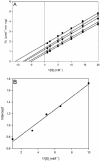
The fluorescent dihydroxyquinoline chromophore of the pyoverdine siderophore in Pseudomonas is a condensation product of D-tyrosine and l-2,4-diaminobutyrate. Both pvdH and asd (encoding aspartate beta-semialdehyde dehydrogenase) knockout mutants of Pseudomonas aeruginosa PAO1 were unable to synthesize pyoverdine under iron-limiting conditions in the absence of l-2,4-diaminobutyrate in the culture media. The pvdH gene was subcloned, and the gene product was hyperexpressed and purified from P. aeruginosa PAO1. PvdH was found to catalyze an aminotransferase reaction, interconverting aspartate beta-semialdehyde and l-2,4-diaminobutyrate. Steady-state kinetic analysis with a novel coupled assay established that the enzyme adopts a ping-pong kinetic mechanism and has the highest specificity for alpha-ketoglutarate. The specificity of the enzyme toward the smaller keto acid pyruvate is 41-fold lower. The enzyme has negligible activity toward other keto acids tested. Homologues of PvdH were present in the genomes of other Pseudomonas spp. These homologues were found in the DNA loci of the corresponding genomes that contain other pyoverdine synthesis genes. This suggests that there is a general mechanism of l-2,4-diaminobutyrate synthesis in Pseudomonas strains that produce the pyoverdine siderophore.
Figures
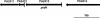
References
-
- Arrio-Dupont, M., P. R. Coulet, and D. C. Gautheron. 1985. Coupled reaction of immobilized aspartate aminotransferase and malate dehydrogenase. A plausible model for the cellular behaviour of these enzymes. Biochim. Biophys. Acta 829:58-68. - PubMed
-
- Böckmann, M., K. Taraz, and H. Budzikiewicz. 1997. Biogenesis of the pyoverdin chromophore. Z. Naturforsch. C 52:319-324.
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Chipperfield, J. R., and C. Ratledge. 2000. Salicylate is not a bacterial siderophore: a theoretical study. Biometals 13:165-168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

