Assembly dynamics of FtsZ rings in Bacillus subtilis and Escherichia coli and effects of FtsZ-regulating proteins
- PMID: 15317782
- PMCID: PMC516820
- DOI: 10.1128/JB.186.17.5775-5781.2004
Assembly dynamics of FtsZ rings in Bacillus subtilis and Escherichia coli and effects of FtsZ-regulating proteins
Abstract
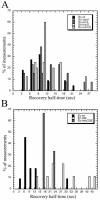
FtsZ is the major cytoskeletal component of the bacterial cell division machinery. It forms a ring-shaped structure (the Z ring) that constricts as the bacterium divides. Previous in vivo experiments with green fluorescent protein-labeled FtsZ and fluorescence recovery after photobleaching have shown that the Escherichia coli Z ring is extremely dynamic, continually remodeling itself with a half time of 30 s, similar to microtubules in the mitotic spindle. In the present work, under different experimental conditions, we have found that the half time for fluorescence recovery of E. coli Z rings is even shorter (approximately 9 s). As before, the turnover appears to be coupled to GTP hydrolysis, since the mutant FtsZ84 protein, with reduced GTPase in vitro, showed an approximately 3-fold longer half time. We have also extended the studies to Bacillus subtilis and found that this species exhibits equally rapid dynamics of the Z ring (half time, approximately 8 s). Interestingly, null mutations of the FtsZ-regulating proteins ZapA, EzrA, and MinCD had only modest effects on the assembly dynamics. This suggests that these proteins do not directly regulate FtsZ subunit exchange in and out of polymers. In B. subtilis, only 30 to 35% of the FtsZ protein was in the Z ring, from which we conclude that a Z ring only 2 or 3 protofilaments thick can function for cell division.
Figures




References
-
- Ben-Yehuda, S., and R. Losick. 2002. Asymmetric cell division in B. subtilis involves a spiral-like intermediate of the cytokinetic protein FtsZ. Cell 109:257-266. - PubMed
-
- Cassimeris, L. U., R. A. Walker, N. K. Pryer, and E. D. Salmon. 1987. Dynamic instability of microtubules. Bioessays 7:149-154. - PubMed
-
- Desai, A., and T. J. Mitchison. 1997. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 13:83-117. - PubMed
-
- Erickson, H. P., and E. T. O'Brien. 1992. Microtubule dynamic instability and GTP hydrolysis. Annu. Rev. Biophys. Struct. Biol. 21:145-166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

