A critical role of tropomyosins in TGF-beta regulation of the actin cytoskeleton and cell motility in epithelial cells
- PMID: 15317845
- PMCID: PMC519159
- DOI: 10.1091/mbc.e04-04-0353
A critical role of tropomyosins in TGF-beta regulation of the actin cytoskeleton and cell motility in epithelial cells
Abstract
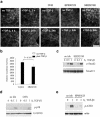
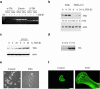
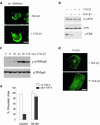
We have investigated transforming growth factor beta (TGF-beta)-mediated induction of actin stress fibers in normal and metastatic epithelial cells. We found that stress fiber formation requires de novo protein synthesis, p38Mapk and Smad signaling. We show that TGF-beta via Smad and p38Mapk up-regulates expression of actin-binding proteins including high-molecular-weight tropomyosins, alpha-actinin and calponin h2. We demonstrate that, among these proteins, tropomyosins are both necessary and sufficient for TGF-beta induction of stress fibers. Silencing of tropomyosins with short interfering RNAs (siRNAs) blocks stress fiber assembly, whereas ectopic expression of tropomyosins results in stress fibers. Ectopic-expression and siRNA experiments show that Smads mediate induction of tropomyosins and stress fibers. Interestingly, TGF-beta induction of stress fibers was not accompanied by changes in the levels of cofilin phosphorylation. TGF-beta induction of tropomyosins and stress fibers are significantly inhibited by Ras-ERK signaling in metastatic breast cancer cells. Inhibition of the Ras-ERK pathway restores TGF-beta induction of tropomyosins and stress fibers and thereby reduces cell motility. These results suggest that induction of tropomyosins and stress fibers play an essential role in TGF-beta control of cell motility, and the loss of this TGF-beta response is a critical step in the acquisition of metastatic phenotype by tumor cells.
Figures






References
-
- Ayscough, K.R. (1998). In vivo functions of actin-binding proteins. Curr. Opin. Cell Biol. 10, 102-111. - PubMed
-
- Bakin, A.V., and Curran, T. (1999). Role of DNA 5-methylcytosine transferase in cell transformation by fos. Science 283, 387-390. - PubMed
-
- Bakin, A.V., Rinehart, C., Tomlinson, A.K., and Arteaga, C.L. (2002). p38 mitogen-activated protein kinase is required for TGF{beta}-mediated fibroblastic transdifferentiation and cell migration. J. Cell Sci. 115, 3193-3206. - PubMed
-
- Bakin, A.V., Tomlinson, A.K., Bhowmick, N.A., Moses, H.L., and Arteaga, C.L. (2000). Phosphatidylinositol 3-kinase function is required for TGFbeta-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 275, 36803-36810. - PubMed
-
- Bamburg, J.R. (1999). Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 15, 185-230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

