Synaptic transformations underlying highly selective auditory representations of learned birdsong
- PMID: 15317851
- PMCID: PMC6729779
- DOI: 10.1523/JNEUROSCI.0947-04.2004
Synaptic transformations underlying highly selective auditory representations of learned birdsong
Abstract
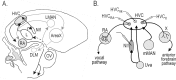
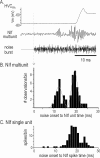
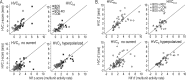
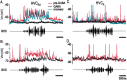
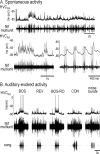
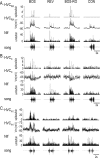
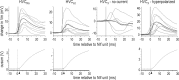
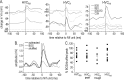
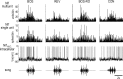
Stimulus-specific neuronal responses are a striking characteristic of several sensory systems, although the synaptic mechanisms underlying their generation are not well understood. The songbird nucleus HVC (used here as a proper name) contains projection neurons (PNs) that fire temporally sparse bursts of action potentials to playback of the bird's own song (BOS) but are essentially silent when presented with other acoustical stimuli. To understand how such remarkable stimulus specificity emerges, it is necessary to compare the auditory-evoked responsiveness of the afferents of HVC with synaptic responses in identified HVC neurons. We found that inactivating the interfacial nucleus of the nidopallium (NIf) could eliminate all auditory-evoked subthreshold activity in both HVC PN types, consistent with NIf serving as the major auditory afferent of HVC. Simultaneous multiunit extracellular recordings in NIf and intracellular recordings in HVC revealed that NIf population activity and HVC subthreshold responses were similar in their selectivity for BOS and that NIf spikes preceded depolarizations in all HVC cell types. These results indicate that information about the BOS as well as other auditory stimuli is transmitted synaptically from NIf to HVC. Unlike HVC PNs, however, HVC-projecting NIf neurons fire throughout playback of BOS as well as non-BOS stimuli. Therefore, temporally sparse BOS-evoked firing and enhanced BOS selectivity, manifested as an absence of suprathreshold responsiveness to non-BOS stimuli, emerge in HVC. The transformation to a sparse auditory representation parallels differences in NIf and HVC activity patterns seen during singing, which may point to a common mechanism for encoding sensory and motor representations of song.
Figures

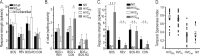

References
-
- Boco T, Margoliash D (2001) NIf is a major source of auditory and spontaneous drive to HVc. Soc Neurosci Abstr 27: 318.2.
-
- Bottjer SW, Halsema KA, Brown SA, Miesner EA (1989) Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol 279: 312-326. - PubMed
-
- Bringuier V, Chavane F, Glaeser L, Fregnac Y (1999) Horizontal propagation of visual activity in the synaptic integration field of area 17 neurons. Science 283: 695-699. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
