Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB
- PMID: 15317858
- PMCID: PMC6729771
- DOI: 10.1523/JNEUROSCI.2111-04.2004
Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB
Abstract
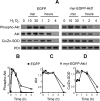
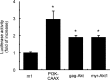
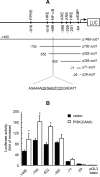
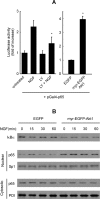
Aerobic cells adjust the expression of antioxidant enzymes to maintain reactive oxygen species within tolerable levels. In addition, phosphatidylinositol 3 kinase (PI3K) and its downstream protein kinase effector Akt adapt cells to survive in the presence of oxidative stress. Here we provide evidence for an association between these two defense systems via transcriptional regulation of Cu/Zn-superoxide dismutase (Cu/Zn-SOD). PC12 pheochromocytoma cells expressing active Akt1 exhibit lower ROS levels in response to hydrogen peroxide, as determined with the superoxide-sensitive probe hydroethidine. Transfection of constitutive or 4-hydroxytamoxifen-inducible versions of Akt1 results in higher messenger RNA and protein levels of Cu/Zn-SOD. Luciferase reporter constructs, carrying different length fragments of the human sod1 gene promoter, have identified a region between -552 and -355 that is targeted by PI3K and Akt and that contains a putative site of regulation by nuclear factor-kappaB (NF-kappaB). Nerve growth factor (NGF) and Akt augment the transactivating activity and produce higher nuclear levels of p65-NF-kappaB. Electrophoretic mobility shift assays indicate that the putative NF-kappaB regulatory sequence binds p65-NF-kappaB more efficiently in nuclear extracts from these cells. A dominant-negative mutant of IkappaBalpha further demonstrates that the PI3K/Akt axis targets the sod1 promoter at the level of the newly characterized NF-kappaB site. These results illustrate a new mechanism by which the PI3K/Akt pathway protects cells against oxidative stress, involving the upregulation of Cu/Zn-SOD gene expression, and the results identify NF-kappaB as a key mediator in the regulation of this gene.
Figures




Similar articles
-
Sustained activation of phosphatidylinositol 3-kinase/Akt/nuclear factor kappaB signaling mediates G protein-coupled delta-opioid receptor gene expression.J Biol Chem. 2006 Feb 10;281(6):3067-74. doi: 10.1074/jbc.M506721200. Epub 2005 Nov 29. J Biol Chem. 2006. PMID: 16316997
-
Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol.J Biol Chem. 2004 Mar 5;279(10):8919-29. doi: 10.1074/jbc.M309660200. Epub 2003 Dec 19. J Biol Chem. 2004. PMID: 14688281
-
Celiprolol activates eNOS through the PI3K-Akt pathway and inhibits VCAM-1 Via NF-kappaB induced by oxidative stress.Hypertension. 2003 Nov;42(5):1004-13. doi: 10.1161/01.HYP.0000097547.35570.70. Epub 2003 Oct 13. Hypertension. 2003. PMID: 14557279
-
Novel Insight into the Effect of Probiotics in the Regulation of the Most Important Pathways Involved in the Pathogenesis of Type 2 Diabetes Mellitus.Probiotics Antimicrob Proteins. 2024 Jun;16(3):829-844. doi: 10.1007/s12602-023-10056-8. Epub 2023 May 10. Probiotics Antimicrob Proteins. 2024. PMID: 37162668 Review.
-
Transcription factor NF-kappaB: friend or foe of neurons?Mol Psychiatry. 1998 Jan;3(1):15-20. doi: 10.1038/sj.mp.4000295. Mol Psychiatry. 1998. PMID: 9491808 Review.
Cited by
-
Phase 0 of the Xenobiotic Response: Nuclear Receptors and Other Transcription Factors as a First Step in Protection from Xenobiotics.Nucl Receptor Res. 2019;6:101447. doi: 10.32527/2019/101447. Epub 2019 Nov 20. Nucl Receptor Res. 2019. PMID: 31815118 Free PMC article.
-
Microglial inflammatory reactions regulated by oxidative stress.J Clin Biochem Nutr. 2023 Jan;72(1):23-27. doi: 10.3164/jcbn.22-71. Epub 2022 Nov 1. J Clin Biochem Nutr. 2023. PMID: 36777074 Free PMC article.
-
DEAD Box Protein Family Member DDX28 Is a Negative Regulator of Hypoxia-Inducible Factor 2α- and Eukaryotic Initiation Factor 4E2-Directed Hypoxic Translation.Mol Cell Biol. 2020 Feb 27;40(6):e00610-19. doi: 10.1128/MCB.00610-19. Print 2020 Feb 27. Mol Cell Biol. 2020. PMID: 31907278 Free PMC article.
-
Antioxidant Activity of Sweet Whey Derived from Bovine, Ovine and Caprine Milk Obtained from Various Small-Scale Cheese Plants in Greece before and after In Vitro Simulated Gastrointestinal Digestion.Antioxidants (Basel). 2023 Aug 27;12(9):1676. doi: 10.3390/antiox12091676. Antioxidants (Basel). 2023. PMID: 37759979 Free PMC article.
-
Transcription factor NFE2L2/NRF2 modulates chaperone-mediated autophagy through the regulation of LAMP2A.Autophagy. 2018;14(8):1310-1322. doi: 10.1080/15548627.2018.1474992. Epub 2018 Jul 26. Autophagy. 2018. PMID: 29950142 Free PMC article.
References
-
- Asanuma M, Hirata H, Cadet JL (1998) Attenuation of 6-hydroxydopamine-induced dopaminergic nigrostriatal lesions in superoxide dismutase transgenic mice. Neuroscience 85: 907-917. - PubMed
-
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857-868. - PubMed
-
- Brunet A, Datta SR, Greenberg ME (2001) Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol 11: 297-305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
