Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation
- PMID: 15317866
- PMCID: PMC6729780
- DOI: 10.1523/JNEUROSCI.1691-04.2004
Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation
Abstract
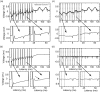
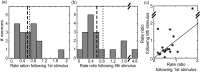
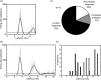
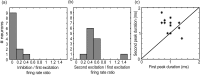
High-frequency stimulation of the globus pallidus (GP) has emerged as a successful tool for treating Parkinson's disease and other motor disorders. However, the mechanism governing its therapeutic effect is still under debate. To shed light on the basic mechanism of deep brain stimulation (DBS), we performed microstimulation in the GP of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated monkey while recording with other microelectrodes in the same nucleus. We used robust methods to reduce the stimulus artifact, and 600-3000 repetitions of a single stimulus and of high-frequency short trains (10-40 stimuli), enabling high temporal resolution analysis of neural responses. Low-frequency stimulation yielded a typical three-stage response: short-term (2-3 msec duration) activity, followed by mid-term (15-25 msec) inhibition, and occasionally longer-term (30-40 msec) excitation. Trains of high-frequency stimuli elicited complex locking of the response to the stimuli in most neurons. The locking displayed a stereotypic temporal structure consisting of three short-duration (1-2 msec) phases: an initial (mean latency = 2.9 msec) excitation followed by an inhibition (4.6 msec) and a second excitation (6.3 msec). The change in the mean firing rate was mixed; the majority of the neurons displayed partial inhibition during the stimulus train. Slow inhibitory and excitatory multiphase changes in the firing rate were observed after the stimulus trains. The activity of neurons recorded simultaneously displayed rate correlations but no spike-to-spike correlations. Our results suggest that the effect of DBS on the GP is not complete inhibition but rather a complex reshaping of the temporal structure of the neuronal activity within that nucleus.
Figures




References
-
- Abeles M, Goldstein MHJ (1977) Multispike train analysis. IEEE Trans Biomed Eng 65: 762-773.
-
- Aertsen AM, Gerstein GL, Habib MK, Palm G (1989) Dynamics of neuronal firing correlation: modulation of “effective connectivity.” J Neurophysiol 61: 900-917. - PubMed
-
- Anderson ME, Postupna N, Ruffo M (2003) Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol 89: 1150-1160. - PubMed
-
- Ashby P, Strafella A, Dostrovsky JO, Lozano A, Lang AE (1998) Immediate motor effects of stimulation through electrodes implanted in the human globus pallidus. Stereotact Funct Neurosurg 70: 1-18. - PubMed
-
- Bar-Gad I, Morris G, Bergman H (2003) Information processing, dimensionality reduction and reinforcement learning in the basal ganglia. Prog Neurobiol 71: 439-473. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
