Sensitization of interferon-gamma induced apoptosis in human osteosarcoma cells by extracellular S100A4
- PMID: 15318945
- PMCID: PMC515304
- DOI: 10.1186/1471-2407-4-52
Sensitization of interferon-gamma induced apoptosis in human osteosarcoma cells by extracellular S100A4
Abstract
Background: S100A4 is a small Ca2+-binding protein of the S100 family with metastasis-promoting properties. Recently, secreted S100A4 protein has been shown to possess a number of functions, including induction of angiogenesis, stimulation of cell motility and neurite extension.
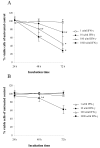
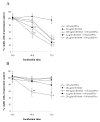
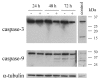
Methods: Cell cultures from two human osteosarcoma cell lines, OHS and its anti-S100A4 ribozyme transfected counterpart II-11b, was treated with IFN-gamma and recombinant S100A4 in order to study the sensitizing effects of extracellular S100A4 on IFN-gamma mediated apoptosis. Induction of apoptosis was demonstrated by DNA fragmentation, cleavage of poly (ADP-ribose) polymerase and Lamin B.
Results: In the present work, we found that the S100A4-expressing human osteosarcoma cell line OHS was more sensitive to IFN-gamma-mediated apoptosis than the II-11b cells. S100A4 protein was detected in conditioned medium from OHS cells, but not from II-11b cells, and addition of recombinant S100A4 to the cell medium sensitized II-11b cells to apoptosis induced by IFN-gamma. The S100A4/IFN-gamma-mediated induction of apoptosis was shown to be independent of caspase activation, but dependent on the formation of reactive oxygen species. Furthermore, addition of extracellular S100A4 was demonstrated to activate nuclear factor-kappa B (NF-kappa B).
Conclusion: In conclusion, we have shown that S100A4 sensitizes osteosarcoma cells to IFN-gamma-mediated induction of apoptosis. Additionally, extracellular S100A4 activates NF-kappa B, but whether these events are causally related remains unknown.
Figures





Similar articles
-
Osteopontin--an important downstream effector of S100A4-mediated invasion and metastasis.Int J Cancer. 2011 Aug 15;129(4):780-90. doi: 10.1002/ijc.25735. Epub 2011 Mar 11. Int J Cancer. 2011. PMID: 20957651
-
Interferon-gamma-induced suppression of S100A4 transcription is mediated by the class II transactivator.Tumour Biol. 2007;28(1):27-35. doi: 10.1159/000097700. Epub 2006 Dec 12. Tumour Biol. 2007. PMID: 17143014
-
S100A4 involvement in metastasis: deregulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in osteosarcoma cells transfected with an anti-S100A4 ribozyme.Cancer Res. 1999 Sep 15;59(18):4702-8. Cancer Res. 1999. PMID: 10493528
-
S100A4 (Mts1): is there any relation to the pathogenesis of rheumatoid arthritis?Autoimmun Rev. 2006 Feb;5(2):129-31. doi: 10.1016/j.autrev.2005.09.010. Epub 2005 Oct 3. Autoimmun Rev. 2006. PMID: 16431343 Review.
-
S100A4: a common mediator of epithelial-mesenchymal transition, fibrosis and regeneration in diseases?J Mol Med (Berl). 2008 May;86(5):507-22. doi: 10.1007/s00109-007-0301-3. Epub 2008 Mar 6. J Mol Med (Berl). 2008. PMID: 18322670 Review.
Cited by
-
S100A4 expression in xenograft tumors of human carcinoma cell lines is induced by the tumor microenvironment.Am J Pathol. 2011 May;178(5):2389-96. doi: 10.1016/j.ajpath.2011.01.022. Am J Pathol. 2011. PMID: 21514449 Free PMC article.
-
S100A4 and metastasis: a small actor playing many roles.Am J Pathol. 2010 Feb;176(2):528-35. doi: 10.2353/ajpath.2010.090526. Epub 2009 Dec 17. Am J Pathol. 2010. PMID: 20019188 Free PMC article. Review.
-
Protective effect of cilostazol and verapamil against thioacetamide-induced hepatotoxicity in rats may involve Nrf2/GSK-3β/NF-κB signaling pathway.Toxicol Res (Camb). 2022 Aug 9;11(5):718-729. doi: 10.1093/toxres/tfac045. eCollection 2022 Oct. Toxicol Res (Camb). 2022. PMID: 36337252 Free PMC article.
-
S100A4 in esophageal cancer: is this the one to blame?World J Gastroenterol. 2012 Aug 14;18(30):3931-5. doi: 10.3748/wjg.v18.i30.3931. World J Gastroenterol. 2012. PMID: 22912541 Free PMC article.
-
Signal transduction mechanisms involved in S100A4-induced activation of the transcription factor NF-kappaB.BMC Cancer. 2010 May 28;10:241. doi: 10.1186/1471-2407-10-241. BMC Cancer. 2010. PMID: 20507646 Free PMC article.
References
-
- Maelandsmo GM, Hovig E, Skrede M, Engebraaten O, Florenes VA, Myklebost O, Grigorian M, Lukanidin E, Scanlon KJ, Fodstad O. Reversal of the in vivo metastatic phenotype of human tumor cells by an anti-CAPL (mts1) ribozyme. Cancer Res. 1996;56:5490–5498. - PubMed
-
- Kriajevska MV, Cardenas MN, Grigorian MS, Ambartsumian NS, Georgiev GP, Lukanidin EM. Non-muscle myosin heavy chain as a possible target for protein encoded by metastasis-related mts-1 gene. J Biol Chem. 1994;269:19679–19682. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

