Cochlear function in Prestin knockout mice
- PMID: 15319415
- PMCID: PMC1665294
- DOI: 10.1113/jphysiol.2004.069559
Cochlear function in Prestin knockout mice
Abstract
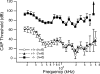
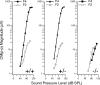
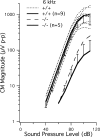
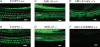
Gross-potential recordings in mice lacking the Prestin gene indicate that compound action potential (CAP) thresholds are shifted by approximately 45 dB at 5 kHz and by approximately 60 dB at 33 kHz. However, in order to conclude that outer hair cell (OHC) electromotility is associated with the cochlear amplifier, frequency selectivity must be evaluated and the integrity of the OHC's forward transducer ascertained. The present report demonstrates no frequency selectivity in CAP tuning curves recorded in homozygotes. In addition, CAP input-output functions indicate that responses in knockout mice approach those in controls at high levels where the amplifier has little influence. Although the cochlear microphonic in knockout mice remains approximately 12 dB below that in wild-type mice even at the highest levels, this deficit is thought to reflect hair cell losses in mice lacking prestin. A change in OHC forward transduction is not implied because knockout mice display non-linear responses similar to those in controls. For example, homozygotes exhibit a bipolar summating potential (SP) with positive responses at high frequencies; negative responses at low frequencies. Measurement of intermodulation distortion also shows that the cubic difference tone, 2f(1)-f(2), is approximately 20 dB down from the primaries in both homozygotes and their controls. Because OHCs are the sole generators of the negative SP and because 2f(1)-f(2) is also thought to originate in OHC transduction, these data support the idea that forward transduction is not degraded in OHCs lacking prestin. Finally, application of AM1-43, which initially enters hair cells through their transducer channels, produces fluorescence in wild-type and knockout mice indicating transducer channel activity in both inner and outer hair cells.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

