Activity-dependent excitability changes in hippocampal CA3 cell Schaffer axons
- PMID: 15319418
- PMCID: PMC1665259
- DOI: 10.1113/jphysiol.2004.071225
Activity-dependent excitability changes in hippocampal CA3 cell Schaffer axons
Abstract
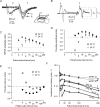
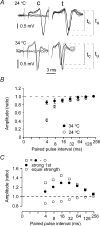
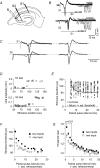
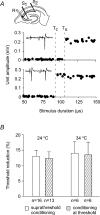
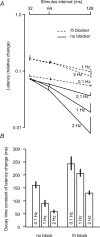
The membrane potential changes following action potentials in thin unmyelinated cortical axons with en passant boutons may be important for synaptic release and conduction abilities of such axons. In the lack of intra-axonal recording techniques we have used extracellular excitability testing as an indirect measure of the after-potentials. We recorded from individual CA3 soma in hippocampal slices and activated the axon with a range of stimulus intensities. When conditioning and test stimuli were given to the same site the excitability changes were partly masked by local effects of the stimulating electrode at intervals < 5 ms. Therefore, we elicited the conditioning action potential from one axonal branch and tested the excitability of another branch. We found that a single action potential reduced the axonal excitability for 15 ms followed by an increased excitability for approximately 200 ms at 24 degrees C. Using field recordings of axonal action potentials we show that raising the temperature to 34 degrees C reduced the magnitude and duration of the initial depression. However, the duration of the increased excitability was very similar (time constant 135 +/- 20 ms) at 24 and 34 degrees C, and with 2.0 and 0.5 mM Ca2+ in the bath. At stimulus rates > 1 Hz, a condition that activates a hyperpolarization-activated current (Ih) in these axons, the decay was faster than at lower stimulation rates. This effect was reduced by the Ih blocker ZD7288. These data suggest that the decay time course of the action potential-induced hyperexcitability is determined by the membrane time constant.
Figures

Similar articles
-
Unmyelinated axons in the rat hippocampus hyperpolarize and activate an H current when spike frequency exceeds 1 Hz.J Physiol. 2003 Oct 15;552(Pt 2):459-70. doi: 10.1113/jphysiol.2003.048058. J Physiol. 2003. PMID: 14561829 Free PMC article.
-
Activity-dependent differences in function between proximal and distal Schaffer collaterals.J Neurophysiol. 2015 Jun 1;113(10):3646-62. doi: 10.1152/jn.00446.2014. Epub 2015 Apr 8. J Neurophysiol. 2015. PMID: 25855695 Free PMC article.
-
Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation.J Neurophysiol. 1996 Sep;76(3):1836-49. doi: 10.1152/jn.1996.76.3.1836. J Neurophysiol. 1996. PMID: 8890296
-
Backpropagation of action potentials generated at ectopic axonal loci: hypothesis that axon terminals integrate local environmental signals.Brain Res Brain Res Rev. 1995 Jul;21(1):42-92. doi: 10.1016/0165-0173(95)00004-m. Brain Res Brain Res Rev. 1995. PMID: 8547954 Review.
-
Excitability tuning of axons in the central nervous system.J Physiol Sci. 2016 May;66(3):189-96. doi: 10.1007/s12576-015-0415-2. Epub 2015 Oct 22. J Physiol Sci. 2016. PMID: 26493201 Free PMC article. Review.
Cited by
-
Comparing the effects of mineral trioxide aggregate and calcium enriched mixture on neuronal cells using an electrophysiological approach.Iran Endod J. 2012 Spring;7(2):79-87. Epub 2012 Jun 1. Iran Endod J. 2012. PMID: 23056124 Free PMC article.
-
Potassium diffusive coupling in neural networks.Philos Trans R Soc Lond B Biol Sci. 2010 Aug 12;365(1551):2347-62. doi: 10.1098/rstb.2010.0050. Philos Trans R Soc Lond B Biol Sci. 2010. PMID: 20603356 Free PMC article. Review.
-
The membrane response of hippocampal CA3b pyramidal neurons near rest: Heterogeneity of passive properties and the contribution of hyperpolarization-activated currents.Neuroscience. 2009 May 5;160(2):359-70. doi: 10.1016/j.neuroscience.2009.01.082. Epub 2009 Feb 13. Neuroscience. 2009. PMID: 19232379 Free PMC article.
-
High-fidelity optical excitation of cortico-cortical projections at physiological frequencies.J Neurophysiol. 2016 Nov 1;116(5):2056-2066. doi: 10.1152/jn.00456.2016. Epub 2016 Aug 3. J Neurophysiol. 2016. PMID: 27489370 Free PMC article.
-
Dynamic synapses as archives of synaptic history: state-dependent redistribution of synaptic efficacy in the rat hippocampal CA1.J Physiol. 2005 Jul 1;566(Pt 1):143-60. doi: 10.1113/jphysiol.2005.086595. Epub 2005 Apr 21. J Physiol. 2005. PMID: 15845579 Free PMC article.
References
-
- Andersen P. Interhippocampal impulses II. Apical dendritic activation of CA1 neurons. Acta Physiol Scand. 1960;48:178–208. - PubMed
-
- Bartesaghi R. Supernormal excitability of fibers of the dorsal hippocampal commissure. Exp Neurol. 1987;96:208–213. - PubMed
-
- Blight AR, Someya S. Depolarizing afterpotentials in myelinated axons of mammalian spinal cord. Neuroscience. 1985;15:1–12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

