Neutralizing antibodies in patients with severe acute respiratory syndrome-associated coronavirus infection
- PMID: 15319862
- PMCID: PMC7199490
- DOI: 10.1086/423286
Neutralizing antibodies in patients with severe acute respiratory syndrome-associated coronavirus infection
Abstract
Background: Severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) is the principal etiologic agent of SARS. We analyzed serum samples obtained from 623 patients with SARS in Beijing, to determine whether infection with SARS-CoV can elicit neutralizing antibodies (NAbs).
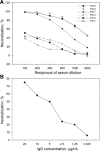
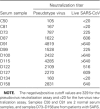
Methods: We developed a highly sensitive and safe neutralization assay using the SARS-CoV pseudotyped virus and used this assay to determine the titers of the NAbs in serum samples from patients with SARS.
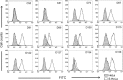
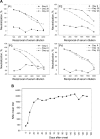
Results: We found that 85.9% of serum samples contained NAbs against SARS-CoV and that most of the NAb activities could be attributed to immunoglobulin G. The NAbs became detectable first at 5-10 days after the onset of symptoms, and their levels peaked at 20-30 days and then were sustained for >150 days. The serum samples could neutralize the pseudotype particles bearing the spike glycoproteins from different SARS-CoV strains, suggesting that the NAbs to SARS-CoV were broadly reactive.
Conclusions: NAbs to SARS-CoV are broadly elicited in patients with SARS and, according to their kinetics, may correlate with viral load during the early stages of the disease. These results suggest that it is possible to develop effective vaccines against SARS and that NAbs provide a potential strategy for treating patients with SARS.
Figures


References
-
- Thiel V, Ivanov KA, Putics A, et al. Mechanisms and enzymes involved in SARS coronavirus genome expression. J Gen Virol. 2003;84:2305–15. - PubMed
-
- Poutanen SM, Low DE, Henry B, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. - PubMed
-
- Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. - PubMed
-
- Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

