Activation of dendritic cells by liposomes prepared from phosphatidylinositol mannosides from Mycobacterium bovis bacillus Calmette-Guerin and adjuvant activity in vivo
- PMID: 15322018
- PMCID: PMC517455
- DOI: 10.1128/IAI.72.9.5235-5246.2004
Activation of dendritic cells by liposomes prepared from phosphatidylinositol mannosides from Mycobacterium bovis bacillus Calmette-Guerin and adjuvant activity in vivo
Abstract
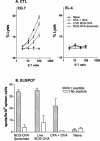
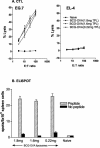
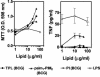
Liposome vesicles could be formed at 65 degrees C from the chloroform-soluble, total polar lipids (TPL) extracted from Mycobacterium bovis bacillus Calmette-Guérin (BCG). Mice immunized with ovalbumin (OVA) entrapped in TPL liposomes produced both anti-OVA antibody and cytotoxic T lymphocyte responses. Murine bone marrow-derived dendritic cells were activated to secrete interleukin-6 (IL-6), IL-12, and tumor necrosis factor upon exposure to antigen-free TPL liposomes. Three phosphoglycolipids and three phospholipids comprising 96% of TPL were identified as phosphatidylinositol dimannoside, palmitoyl-phosphatidylinositol dimannoside, dipalmitoyl-phosphatidylinositol dimannoside, phosphatidylinositol, phosphatidylethanolamine, and cardiolipin. The activation of dendritic cells by liposomes prepared from each purified lipid component of TPL was evaluated in vitro. A basal activity of phosphatidylinositol liposomes to activate proinflammatory cytokine production appeared to be attributable to the tuberculosteric fatty acyl 19:0 chain characteristic of mycobacterial glycerolipids, as similar lipids lacking tuberculosteric chains showed little activity. Phosphatidylinositol dimannoside was identified as the primary lipid that activated dendritic cells to produce amounts of proinflammatory cytokines several times higher than the basal level, indicating the importance of mannose residues. Although the activity of phosphatidylinositol dimannoside was little influenced by palmitoylation of mannose at C-6, a further palmitoylation at inositol C-3 diminished the induction levels of IL-6 and IL-12. Further, OVA entrapped in palmitoyl-phosphatidylinositol dimannoside liposomes was delivered to dendritic cells for major histocompatibility complex class I presentation more effectively than TPL OVA-liposomes. BCG liposomes containing mannose lipids caused up-regulation of costimulatory molecules and CD40. Thus, the inclusion of pure phosphatidylinositol mannosides of BCG in lipid vesicle vaccines represents a simple and efficient option for targeting antigen delivery and providing immune stimulation.
Figures



Similar articles
-
Liposome adjuvants prepared from the total polar lipids of Haloferax volcanii, Planococcus spp. and Bacillus firmus differ in ability to elicit and sustain immune responses.Vaccine. 2004 Jun 2;22(17-18):2154-62. doi: 10.1016/j.vaccine.2003.11.054. Vaccine. 2004. PMID: 15149772
-
Immunostimulatory and antigen delivery properties of liposomes made up of total polar lipids from non-pathogenic bacteria leads to efficient induction of both innate and adaptive immune responses.Vaccine. 2011 Mar 16;29(13):2381-91. doi: 10.1016/j.vaccine.2011.01.110. Epub 2011 Feb 5. Vaccine. 2011. PMID: 21300103
-
Phosphatidylinositol mannosides are efficient mucosal adjuvants.Immunol Invest. 2008;37(2):129-42. doi: 10.1080/08820130701690782. Immunol Invest. 2008. PMID: 18300038
-
Targeting dendritic cells with antigen-containing liposomes: antitumour immunity.Expert Opin Biol Ther. 2004 Nov;4(11):1735-47. doi: 10.1517/14712598.4.11.1735. Expert Opin Biol Ther. 2004. PMID: 15500402 Review.
-
Oligomannose-coated liposome as a novel adjuvant for the induction of cellular immune responses to control disease status.Biomed Res Int. 2013;2013:562924. doi: 10.1155/2013/562924. Epub 2013 Oct 10. Biomed Res Int. 2013. PMID: 24224170 Free PMC article. Review.
Cited by
-
Mycobacterial outer membrane is a lipid bilayer and the inner membrane is unusually rich in diacyl phosphatidylinositol dimannosides.Proc Natl Acad Sci U S A. 2014 Apr 1;111(13):4958-63. doi: 10.1073/pnas.1403078111. Epub 2014 Mar 17. Proc Natl Acad Sci U S A. 2014. PMID: 24639491 Free PMC article.
-
Ac2PIM-responsive miR-150 and miR-143 target receptor-interacting protein kinase 2 and transforming growth factor beta-activated kinase 1 to suppress NOD2-induced immunomodulators.J Biol Chem. 2015 Oct 30;290(44):26576-86. doi: 10.1074/jbc.M115.662817. Epub 2015 Sep 21. J Biol Chem. 2015. PMID: 26391398 Free PMC article.
-
Generation of Liposomes to Study the Effect of Mycobacterium Tuberculosis Lipids on HIV-1 cis- and trans-Infections.Int J Mol Sci. 2021 Feb 16;22(4):1945. doi: 10.3390/ijms22041945. Int J Mol Sci. 2021. PMID: 33669411 Free PMC article.
-
Novel adjuvant formulations for delivery of anti-tuberculosis vaccine candidates.Adv Drug Deliv Rev. 2016 Jul 1;102:73-82. doi: 10.1016/j.addr.2015.11.012. Epub 2015 Nov 17. Adv Drug Deliv Rev. 2016. PMID: 26596558 Free PMC article. Review.
-
Cationic liposomes containing mycobacterial lipids: a new powerful Th1 adjuvant system.Infect Immun. 2005 Sep;73(9):5817-26. doi: 10.1128/IAI.73.9.5817-5826.2005. Infect Immun. 2005. PMID: 16113300 Free PMC article.
References
-
- Barratt, G., J. P. Tenu, A. Yapo, and J. F. Petit. 1986. Preparation and characterisation of liposomes containing mannosylated phospholipids capable of targetting drugs to macrophages. Biochim. Biophys. Acta 862:153-164. - PubMed
-
- Besra, G. S., and P. J. Brennan. 1994. The glycolipids of mycobacteria, p. 203-232. In C. Fenselau (ed.), Mass spectrometry for the characterization of microorganisms. Oxford University Press, New York, N.Y.
-
- Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Med. Sci. 37:911-917. - PubMed
-
- Brennan, P., and C. E. Ballou. 1967. Biosynthesis of mannophosphoinositides by Mycobacterium phlei. The family of dimannophosphoinositides. J. Biol. Chem. 242:3046-3056. - PubMed
-
- Campos, M. A., I. C. Almeida, O. Takeuchi, S. Akira, E. P. Valente, D. O. Procopio, L. R. Travassos, J. A. Smith, D. T. Golenbock, and R. T. Gazzinelli. 2001. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167:416-423. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

