Involvement of the lectin pathway of complement activation in antimicrobial immune defense during experimental septic peritonitis
- PMID: 15322019
- PMCID: PMC517465
- DOI: 10.1128/IAI.72.9.5247-5252.2004
Involvement of the lectin pathway of complement activation in antimicrobial immune defense during experimental septic peritonitis
Abstract
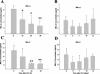
A critical first line of defense against infection is constituted by the binding of natural antibodies to microbial surfaces, activating the complement system via the classical complement activation pathway. In this function, the classical activation pathway is supported and amplified by two antibody-independent complement activation routes, i.e., the lectin pathway and the alternative pathway. We studied the contribution of the different complement activation pathways in the host defense against experimental polymicrobial peritonitis induced by cecal ligation and puncture by using mice deficient in either C1q or factors B and C2. The C1q-deficient mice lack the classical complement activation pathway. While infection-induced mortality of wild-type mice was 27%, mortality of C1q-deficient mice was increased to 60%. Mice with a deficiency of both factors B and C2 lack complement activation via the classical, the alternative, and the lectin pathways and exhibit a mortality of 92%, indicating a significant contribution of the lectin and alternative pathways of complement activation to survival. For 14 days after infection, mannan-binding lectin (MBL)-dependent activation of C4 was compromised. Serum MBL-A and MBL-C levels were significantly reduced for 1 week, possibly due to consumption. mRNA expression profiles did not lend support for either of the two MBL genes to respond as typical acute-phase genes. Our results demonstrate a long-lasting depletion of MBL-A and MBL-C from serum during microbial infection and underline the importance of both the lectin and the alternative pathways for antimicrobial immune defense.
Figures


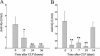
Similar articles
-
Role of the classical pathway of complement activation in experimentally induced polymicrobial peritonitis.Infect Immun. 2001 Dec;69(12):7304-9. doi: 10.1128/IAI.69.12.7304-7309.2001. Infect Immun. 2001. PMID: 11705901 Free PMC article.
-
Increased susceptibility of complement factor B/C2 double knockout mice and mannan-binding lectin knockout mice to systemic infection with Candida albicans.Mol Immunol. 2008 Sep;45(15):3934-41. doi: 10.1016/j.molimm.2008.06.021. Epub 2008 Jul 30. Mol Immunol. 2008. PMID: 18672286
-
Antibody-mediated activation of the classical pathway of complement may compensate for mannose-binding lectin deficiency.Eur J Immunol. 2004 Sep;34(9):2589-98. doi: 10.1002/eji.200324401. Eur J Immunol. 2004. PMID: 15307191
-
Interaction of C1q and mannan-binding lectin with viruses.Immunobiology. 2002 Sep;205(4-5):563-74. doi: 10.1078/0171-2985-00155. Immunobiology. 2002. PMID: 12396016 Review.
-
Impact of mannose-binding lectin on susceptibility to infectious diseases.Clin Infect Dis. 2003 Dec 1;37(11):1496-505. doi: 10.1086/379324. Epub 2003 Nov 6. Clin Infect Dis. 2003. PMID: 14614673 Review.
Cited by
-
Epigallocatechin Gallate (EGCG) Promotes the Immune Function of Ileum in High Fat Diet Fed Mice by Regulating Gut Microbiome Profiling and Immunoglobulin Production.Front Nutr. 2021 Sep 20;8:720439. doi: 10.3389/fnut.2021.720439. eCollection 2021. Front Nutr. 2021. PMID: 34616764 Free PMC article.
-
Unraveling the Intricate Web: Complement Activation Shapes the Pathogenesis of Sepsis-Induced Coagulopathy.J Innate Immun. 2024;16(1):337-353. doi: 10.1159/000539502. Epub 2024 May 31. J Innate Immun. 2024. PMID: 38815564 Free PMC article. Review.
-
Phytol-based novel adjuvants in vaccine formulation: 2. Assessment of efficacy in the induction of protective immune responses to lethal bacterial infections in mice.J Immune Based Ther Vaccines. 2006 Oct 23;4:5. doi: 10.1186/1476-8518-4-5. J Immune Based Ther Vaccines. 2006. PMID: 17059608 Free PMC article.
-
Distinct different contributions of the alternative and classical complement activation pathway for the innate host response during sepsis.J Immunol. 2011 Mar 1;186(5):3066-75. doi: 10.4049/jimmunol.1002741. Epub 2011 Jan 24. J Immunol. 2011. PMID: 21263075 Free PMC article.
-
Association between serum ficolin-1 level and disease progression in primary biliary cholangitis.PLoS One. 2020 Sep 11;15(9):e0238300. doi: 10.1371/journal.pone.0238300. eCollection 2020. PLoS One. 2020. PMID: 32915797 Free PMC article.
References
-
- Ambrus, G., P. Gal, M. Kojima, K. Szilagyi, J. Balczer, J. Antal, L. Graf, A. Laich, B. E. Moffatt, W. Schwaeble, R. B. Sim, and P. Zavodszky. 2003. Natural substrates and inhibitors of mannan-binding lectin-associated serine protease-1 and -2: a study on recombinant catalytic fragments. J. Immunol. 170:1374-1382. - PubMed
-
- Botto, M., C. Dell'Agnola, A. E. Bygrave, E. M. Thompson, H. T. Cook, F. Petry, M. Loos, P. P. Pandolfi, and M. J Walport. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19:56-59. - PubMed
-
- Brown, J. S., T. Hussell, S. M. Gilliland, D. W. Holden, J. C. Paton, M. R. Ehrenstein, M. J. Walport, and M. Botto. 2002. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. USA 99:16969-16974. - PMC - PubMed
-
- Chirgwin, J., A. Przybyla, R. MacDonald, and W. Rutter. 1979. Isolation of biologically active ribonucleic acid from sources enriched in ribonucleases. Biochemistry 18:5294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

