Protein patterns at lipid bilayer junctions
- PMID: 15322273
- PMCID: PMC516475
- DOI: 10.1073/pnas.0402168101
Protein patterns at lipid bilayer junctions
Abstract
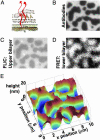
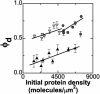
We introduce a simple intermembrane junction system in which to explore pattern and structure formation by membrane-bound proteins. The junction consists of a planar lipid bilayer to which one species of protein (an IgG antibody) is bound, forming a 2D, compressible fluid. Upon the adhesion of a second lipid bilayer, the formerly uniformly distributed proteins rapidly reorganize into patterns of dense and sparse zones. Using a combination of complementary imaging techniques (fluorescence microscopy, fluorescence interference contrast microscopy, and fluorescence resonance energy transfer), we reconstruct the 3D structure of these intermembrane patterns with nanometer-scale topographic resolution, revealing the orientation of the proteins. The patterns form as the rapid bilayer-bilayer adhesion, often radiating outward from an initial, circular contact site, pushes aside the antibodies, sweeping them into areas of high density and clearing low-density regions. Coarsening of these local features is energetically costly and therefore kinetically trapped; the patterns do not change over tens of minutes. These studies demonstrate that membrane mechanical forces alone, i.e., in the absence of specific biochemical interactions, can drive microm-scale organization of membrane proteins.
Copyright 2004 The National Academy of Sciencs of the USA
Figures



References
-
- Dustin, M. L. & Colman, D. R. (2002) Science 29, 785–789. - PubMed
-
- Monks, C. R. F., Freiberg, B. A., Kupfer, H., Sciaky, N. & Kupfer, A. (1998) Nature 395, 82–86. - PubMed
-
- Grakoui, A., Bromley, S. K., Sumen, C., Davis, M. M., Shaw, A. S., Allen, P. M. & Dustin, M. L. (1999) Science 285, 221–227. - PubMed
-
- Lee, K.-H., Dinner, A. R., Tu, C., Campi, G., Raychaudhuri, S., Varma, R., Sims, T. N., Burack, W. R., Wu, H., Wang, J., et al. (2003) Science 302, 1218–1222. - PubMed
-
- Igakura, T., Stinchcombe, J. C., Goon, P. K. C, Taylor, G. P., Weber, J. N., Griffiths, G. M., Tanaka, Y., Osame, M. & Bangham, C. R. M. (2003) Science 299, 1713–1716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

