RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR)
- PMID: 15322274
- PMCID: PMC516517
- DOI: 10.1073/pnas.0402388101
RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR)
Abstract
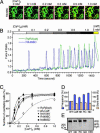
The cardiac ryanodine receptor (RyR2) governs the release of Ca2+ from the sarcoplasmic reticulum, which initiates muscle contraction. Mutations in RyR2 have been linked to ventricular tachycardia (VT) and sudden death, but the precise molecular mechanism is unclear. It is known that when the sarcoplasmic reticulum store Ca2+ content reaches a critical level, spontaneous Ca2+ release occurs, a process we refer to as store-overload-induced Ca2+ release (SOICR). In view of the well documented arrhythmogenic nature of SOICR, we characterized the effects of disease-causing RyR2 mutations on SOICR in human embryonic kidney (HEK)293 cells and found that, at elevated extracellular Ca2+ levels, HEK293 cells expressing RyR2 displayed SOICR in a manner virtually identical to that observed in cardiac cells. Using this cell model, we demonstrated that the RyR2 mutations linked to VT and sudden death, N4104K, R4496C, and N4895D, markedly increased the occurrence of SOICR. At the molecular level, we showed that these RyR2 mutations increased the sensitivity of single RyR2 channels to activation by luminal Ca2+ and enhanced the basal level of [3H]ryanodine binding. We conclude that disease-causing RyR2 mutations, by enhancing RyR2 luminal Ca2+ activation, reduce the threshold for SOICR, which in turn increases the propensity for triggered arrhythmia. Abnormal RyR2 luminal Ca2+ activation likely contributes to the enhanced SOICR commonly observed in various cardiac conditions, including heart failure, and may represent a unifying mechanism for Ca2+ overload-associated VT.
Copyright 2004 The National Academy of Sciencs of the USA
Figures



Similar articles
-
Ryanodine receptor-mediated arrhythmias and sudden cardiac death.Pharmacol Ther. 2009 Aug;123(2):151-77. doi: 10.1016/j.pharmthera.2009.03.006. Epub 2009 Apr 1. Pharmacol Ther. 2009. PMID: 19345240 Free PMC article. Review.
-
Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death.Circ Res. 2005 Nov 25;97(11):1173-81. doi: 10.1161/01.RES.0000192146.85173.4b. Epub 2005 Oct 20. Circ Res. 2005. PMID: 16239587
-
The CPVT-associated RyR2 mutation G230C enhances store overload-induced Ca2+ release and destabilizes the N-terminal domains.Biochem J. 2013 Aug 15;454(1):123-31. doi: 10.1042/BJ20130594. Biochem J. 2013. PMID: 23746327
-
Endoplasmic reticulum Ca2+ measurements reveal that the cardiac ryanodine receptor mutations linked to cardiac arrhythmia and sudden death alter the threshold for store-overload-induced Ca2+ release.Biochem J. 2008 May 15;412(1):171-8. doi: 10.1042/BJ20071287. Biochem J. 2008. PMID: 18092949
-
Catecholaminergic polymorphic ventricular tachycardia: recent mechanistic insights.Cardiovasc Res. 2005 Aug 15;67(3):379-87. doi: 10.1016/j.cardiores.2005.04.027. Cardiovasc Res. 2005. PMID: 15913575 Review.
Cited by
-
Abnormal termination of Ca2+ release is a common defect of RyR2 mutations associated with cardiomyopathies.Circ Res. 2012 Mar 30;110(7):968-77. doi: 10.1161/CIRCRESAHA.111.256560. Epub 2012 Feb 28. Circ Res. 2012. PMID: 22374134 Free PMC article.
-
Identification of loss-of-function RyR2 mutations associated with idiopathic ventricular fibrillation and sudden death.Biosci Rep. 2021 Apr 30;41(4):BSR20210209. doi: 10.1042/BSR20210209. Biosci Rep. 2021. PMID: 33825858 Free PMC article.
-
Calcium signaling phenomena in heart diseases: a perspective.Mol Cell Biochem. 2007 Apr;298(1-2):1-40. doi: 10.1007/s11010-006-9355-8. Epub 2006 Nov 21. Mol Cell Biochem. 2007. PMID: 17119849 Review.
-
A domain peptide of the cardiac ryanodine receptor regulates channel sensitivity to luminal Ca2+ via cytoplasmic Ca2+ sites.Eur Biophys J. 2008 Apr;37(4):455-67. doi: 10.1007/s00249-007-0238-z. Epub 2007 Nov 24. Eur Biophys J. 2008. PMID: 18038129
-
Ryanodine receptor-mediated arrhythmias and sudden cardiac death.Pharmacol Ther. 2009 Aug;123(2):151-77. doi: 10.1016/j.pharmthera.2009.03.006. Epub 2009 Apr 1. Pharmacol Ther. 2009. PMID: 19345240 Free PMC article. Review.
References
-
- Nuss, H. B., Kaab, S., Kass, D. A., Tomaselli, G. F. & Marban, E. (1999) Am. J. Physiol. 277, H80-H91. - PubMed
-
- Zaugg, C. & Buser, P. (2001) Croat. Med. J. 42, 24-32. - PubMed
-
- Janse, M. (2004) Cardiovasc. Res. 61, 208-217. - PubMed
-
- Pogwizd, S. M. & Bers, D. M. (2004) Trends Cardiovasc. Med. 14, 61-66. - PubMed
-
- Leenhardt, A., Lucet, V., Denjoy, I., Grau, F., Ngoc, D. D. & Coumel, P. (1995) Circulation 91, 1512-1519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

