Asynchronous replication timing of telomeres at opposite arms of mammalian chromosomes
- PMID: 15322275
- PMCID: PMC516496
- DOI: 10.1073/pnas.0404106101
Asynchronous replication timing of telomeres at opposite arms of mammalian chromosomes
Abstract
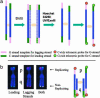
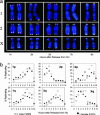
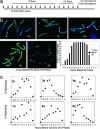
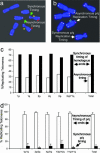
Telomeres are defining structural elements of all linear chromosomes, yet information concerning the timing of their replication in higher eukaryotes is surprisingly limited. We developed an approach that allowed a study of telomere replication patterns of specific mammalian chromosomes. In the Indian muntjac (Muntiacus muntjac), replication timing between respective telomeres of homologous chromosomes was highly coordinated, but no such synchrony was evident for p- and q-arm telomeres of the same chromosome. This finding contrasts with the coordinated timing of both ends of each chromosome in yeast. Also in contrast to yeast, where replication of all telomeres is confined to late S phase, we found specific telomeres in Indian muntjac chromosomes that replicated early in S and other telomeres that replicated later. Finally, replication timing of some but not all telomeres was influenced by telomere length. Knowledge of telomere replication timing represents a first step toward understanding the relationship between telomere replication and telomerase action. The approach, which we call replicative detargeting fluorescence in situ hybridization, is widely applicable to different species and genetic loci.
Copyright 2004 The National Academy of Sciencs of the USA
Figures
Similar articles
-
Human telomerase can immortalize Indian muntjac cells.Exp Cell Res. 2002 Nov 15;281(1):63-76. doi: 10.1006/excr.2002.5645. Exp Cell Res. 2002. PMID: 12441130
-
Replication timing of human telomeres is chromosome arm-specific, influenced by subtelomeric structures and connected to nuclear localization.PLoS Genet. 2010 Apr 22;6(4):e1000920. doi: 10.1371/journal.pgen.1000920. PLoS Genet. 2010. PMID: 20421929 Free PMC article.
-
Characterization of the telomere complex, TERF1 and TERF2 genes in muntjac species with fusion karyotypes.Exp Cell Res. 2005 May 15;306(1):64-74. doi: 10.1016/j.yexcr.2005.02.001. Exp Cell Res. 2005. PMID: 15878333
-
Replication of telomeres and the regulation of telomerase.Cold Spring Harb Perspect Biol. 2013 May 1;5(5):a010405. doi: 10.1101/cshperspect.a010405. Cold Spring Harb Perspect Biol. 2013. PMID: 23543032 Free PMC article. Review.
-
Telomere-binding factors in the regulation of DNA replication.Genes Genet Syst. 2018 Jan 20;92(3):119-125. doi: 10.1266/ggs.17-00008. Epub 2017 Jun 30. Genes Genet Syst. 2018. PMID: 28674277 Review.
Cited by
-
Replication Timing of Human Telomeres is Conserved during Immortalization and Influenced by Respective Subtelomeres.Sci Rep. 2016 Sep 2;6:32510. doi: 10.1038/srep32510. Sci Rep. 2016. PMID: 27587191 Free PMC article.
-
Cell proliferation in the presence of telomerase.PLoS One. 2009;4(2):e4622. doi: 10.1371/journal.pone.0004622. Epub 2009 Feb 27. PLoS One. 2009. PMID: 19247450 Free PMC article.
-
DNA combing reveals intrinsic temporal disorder in the replication of yeast chromosome VI.J Mol Biol. 2008 Jan 4;375(1):12-9. doi: 10.1016/j.jmb.2007.10.046. Epub 2007 Oct 23. J Mol Biol. 2008. PMID: 17999930 Free PMC article. Review.
-
Cell cycle-regulated trafficking of human telomerase to telomeres.Mol Biol Cell. 2006 Feb;17(2):955-65. doi: 10.1091/mbc.e05-09-0903. Epub 2005 Dec 7. Mol Biol Cell. 2006. PMID: 16339074 Free PMC article.
-
Oxidative Stress Induces Telomere Dysfunction and Senescence by Replication Fork Arrest.Cells. 2019 Jan 3;8(1):19. doi: 10.3390/cells8010019. Cells. 2019. PMID: 30609792 Free PMC article.
References
-
- Blackburn, E. H. (2000) Nature 408, 53–56. - PubMed
-
- Blackburn, E. H. (2001) Cell 106, 661–673. - PubMed
-
- Ferreira, M. G., Miller, K. M. & Cooper, J. P. (2004) Mol. Cell 13, 7–18. - PubMed
-
- Watson, J. D. (1972) Nat. New Biol. 239, 197–201. - PubMed
-
- Olovnikov, A. M. (1973) J. Theor. Biol. 41, 181–190. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

