An enzymatic molten globule: efficient coupling of folding and catalysis
- PMID: 15322276
- PMCID: PMC516485
- DOI: 10.1073/pnas.0404109101
An enzymatic molten globule: efficient coupling of folding and catalysis
Abstract
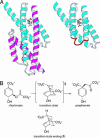
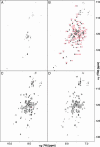
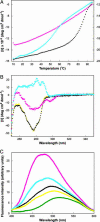
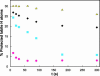
A highly active, monomeric chorismate mutase, obtained by topological redesign of a dimeric helical bundle enzyme from Methanococcus jannaschii, was investigated by NMR and various other biochemical techniques, including H/D exchange. Although structural disorder is generally considered to be incompatible with efficient catalysis, the monomer, unlike its natural counterpart, unexpectedly possesses all of the characteristics of a molten globule. Global conformational ordering, observed upon binding of a transition state analog, indicates that folding can be coupled to catalysis with minimal energetic penalty. These results support the suggestion that many modern enzymes might have evolved from molten globule precursors. Insofar as their structural plasticity confers relaxed substrate specificity and/or catalytic promiscuity, molten globules may also be attractive starting points for the evolution of new catalysts in the laboratory.
Copyright 2004 The National Academy of Sciencs of the USA
Figures
Similar articles
-
Kinetics and thermodynamics of ligand binding to a molten globular enzyme and its native counterpart.J Mol Biol. 2008 Oct 17;382(4):971-7. doi: 10.1016/j.jmb.2008.07.049. Epub 2008 Jul 25. J Mol Biol. 2008. PMID: 18680748
-
Structure and dynamics of a molten globular enzyme.Nat Struct Mol Biol. 2007 Dec;14(12):1202-6. doi: 10.1038/nsmb1325. Epub 2007 Nov 11. Nat Struct Mol Biol. 2007. PMID: 17994104
-
On the relationship between folding and chemical landscapes in enzyme catalysis.Proc Natl Acad Sci U S A. 2008 Sep 16;105(37):13877-82. doi: 10.1073/pnas.0803405105. Epub 2008 Sep 8. Proc Natl Acad Sci U S A. 2008. PMID: 18779576 Free PMC article.
-
Deciphering enzymes. Genetic selection as a probe of structure and mechanism.Eur J Biochem. 2004 May;271(9):1630-7. doi: 10.1111/j.1432-1033.2004.04073.x. Eur J Biochem. 2004. PMID: 15096202 Review.
-
The molten globule state of alpha-lactalbumin.FASEB J. 1996 Jan;10(1):102-9. doi: 10.1096/fasebj.10.1.8566530. FASEB J. 1996. PMID: 8566530 Review.
Cited by
-
Simultaneous optimization of enzyme activity and quaternary structure by directed evolution.Protein Sci. 2005 Aug;14(8):2103-14. doi: 10.1110/ps.051431605. Epub 2005 Jun 29. Protein Sci. 2005. PMID: 15987889 Free PMC article.
-
What Drives Chorismate Mutase to Top Performance? Insights from a Combined In Silico and In Vitro Study.Biochemistry. 2023 Feb 7;62(3):782-796. doi: 10.1021/acs.biochem.2c00635. Epub 2023 Jan 27. Biochemistry. 2023. PMID: 36705397 Free PMC article.
-
Electrostatic transition state stabilization rather than reactant destabilization provides the chemical basis for efficient chorismate mutase catalysis.Proc Natl Acad Sci U S A. 2014 Dec 9;111(49):17516-21. doi: 10.1073/pnas.1408512111. Epub 2014 Nov 24. Proc Natl Acad Sci U S A. 2014. PMID: 25422475 Free PMC article.
-
Catalytically active alkaline molten globular enzyme: Effect of pH and temperature on the structural integrity of 5-aminolevulinate synthase.Biochim Biophys Acta. 2014 Dec;1844(12):2145-54. doi: 10.1016/j.bbapap.2014.09.013. Epub 2014 Sep 18. Biochim Biophys Acta. 2014. PMID: 25240868 Free PMC article.
-
The EVB as a quantitative tool for formulating simulations and analyzing biological and chemical reactions.Faraday Discuss. 2010;145:71-106. doi: 10.1039/B907354J. Faraday Discuss. 2010. PMID: 25285029 Free PMC article.
References
-
- Dyson, H. J. & Wright, P. E. (2002) Curr. Opin. Struct. Biol. 12, 54–60. - PubMed
-
- James, L. C., Roversi, P. & Tawfik, D. S. (2003) Science 299, 1362–1367. - PubMed
-
- Hammes, G. G. (2002) Biochemistry 41, 8221–8228. - PubMed
-
- Dobson, C. M. (1999) Trends Biochem. Sci. 24, 329–332. - PubMed
-
- Ptitsyn, O. B., Pain, R. H., Semisotnov, G. V., Zerovnik, E. & Razgulyaev, O. I. (1990) FEBS Lett. 262, 20–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

