Conservation of functional domain structure in bicarbonate-regulated "soluble" adenylyl cyclases in bacteria and eukaryotes
- PMID: 15322879
- PMCID: PMC3644946
- DOI: 10.1007/s00427-004-0432-2
Conservation of functional domain structure in bicarbonate-regulated "soluble" adenylyl cyclases in bacteria and eukaryotes
Abstract
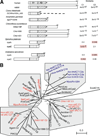
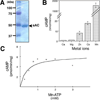
Soluble adenylyl cyclase (sAC) is an evolutionarily conserved bicarbonate sensor. In mammals, it is responsible for bicarbonate-induced, cAMP-dependent processes in sperm required for fertilization and postulated to be involved in other bicarbonate- and carbon dioxide-dependent functions throughout the body. Among eukaryotes, sAC-like cyclases have been detected in mammals and in the fungi Dictyostelium; these enzymes display extensive similarity extending through two cyclase catalytic domains and a long carboxy terminal extension. sAC-like cyclases are also found in a number of bacterial phyla (Cyanobacteria, Actinobacteria, and Proteobacteria), but these enzymes generally possess only a single catalytic domain and little, if any, homology with the remainder of the mammalian protein. Database mining through a number of recently sequenced genomes identified sAC orthologues in additional metazoan phyla (Arthropoda and Chordata) and additional bacterial phyla (Chloroflexi). Interestingly, the Chloroflexi sAC-like cyclases, a family of three enzymes from the thermophilic eubacterium, Chloroflexus aurantiacus, are more similar to eukaryotic sAC-like cyclases (i.e., mammalian sAC and Dictyostelium SgcA) than they are to other bacterial adenylyl cyclases (ACs) (i.e., from Cyanobacteria). The Chloroflexus sAC-like cyclases each possess two cyclase catalytic domains and extensive similarity with mammalian enzymes through their carboxy termini. We cloned one of the Chloroflexus sAC-like cyclases and confirmed it to be stimulated by bicarbonate. These data extend the family of organisms possessing bicarbonate-responsive ACs to numerous phyla within the bacterial and eukaryotic kingdoms.
Figures

Similar articles
-
Structural analysis of human soluble adenylyl cyclase and crystal structures of its nucleotide complexes-implications for cyclase catalysis and evolution.FEBS J. 2014 Sep;281(18):4151-64. doi: 10.1111/febs.12913. Epub 2014 Jul 28. FEBS J. 2014. PMID: 25040695
-
Structure, mechanism, and regulation of soluble adenylyl cyclases - similarities and differences to transmembrane adenylyl cyclases.Biochim Biophys Acta. 2014 Dec;1842(12 Pt B):2535-47. doi: 10.1016/j.bbadis.2014.08.012. Epub 2014 Sep 2. Biochim Biophys Acta. 2014. PMID: 25193033 Review.
-
Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor.Science. 2000 Jul 28;289(5479):625-8. doi: 10.1126/science.289.5479.625. Science. 2000. PMID: 10915626
-
Deducing the origin of soluble adenylyl cyclase, a gene lost in multiple lineages.Mol Biol Evol. 2002 Dec;19(12):2239-46. doi: 10.1093/oxfordjournals.molbev.a004047. Mol Biol Evol. 2002. PMID: 12446814
-
Bicarbonate-regulated soluble adenylyl cyclase.JOP. 2001 Jul;2(4 Suppl):154-8. JOP. 2001. PMID: 11875252 Review.
Cited by
-
Characterization of Plasmodium falciparum adenylyl cyclase-β and its role in erythrocytic stage parasites.PLoS One. 2012;7(6):e39769. doi: 10.1371/journal.pone.0039769. Epub 2012 Jun 26. PLoS One. 2012. PMID: 22761895 Free PMC article.
-
Physiological sensing of carbon dioxide/bicarbonate/pH via cyclic nucleotide signaling.Sensors (Basel). 2011;11(2):2112-28. doi: 10.3390/s110202112. Sensors (Basel). 2011. PMID: 21544217 Free PMC article. Review.
-
Cyanobacteriochrome-based photoswitchable adenylyl cyclases (cPACs) for broad spectrum light regulation of cAMP levels in cells.J Biol Chem. 2018 Jun 1;293(22):8473-8483. doi: 10.1074/jbc.RA118.002258. Epub 2018 Apr 9. J Biol Chem. 2018. PMID: 29632072 Free PMC article.
-
Stimulation of mammalian G-protein-responsive adenylyl cyclases by carbon dioxide.J Biol Chem. 2009 Jan 9;284(2):784-91. doi: 10.1074/jbc.M807239200. Epub 2008 Nov 13. J Biol Chem. 2009. PMID: 19008230 Free PMC article.
-
The central role of cAMP in regulating Plasmodium falciparum merozoite invasion of human erythrocytes.PLoS Pathog. 2014 Dec 18;10(12):e1004520. doi: 10.1371/journal.ppat.1004520. eCollection 2014 Dec. PLoS Pathog. 2014. PMID: 25522250 Free PMC article.
References
-
- Braun T. The effect of divalent cations on bovine spermatozoal adenylate cyclase activity. J Cyclic Nucleotide Res. 1975;1:271–281. - PubMed
-
- Cann MJ, Hammer A, Zhou J, Kanacher T. A defined subset of adenylyl cyclases is regulated by bicarbonate ion. J Biol Chem. 2003;278:35033–35038. - PubMed
-
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

