Efficient synthesis and photodynamic activity of porphyrin-saccharide conjugates: targeting and incapacitating cancer cells
- PMID: 15323552
- PMCID: PMC6180334
- DOI: 10.1021/bi049272v
Efficient synthesis and photodynamic activity of porphyrin-saccharide conjugates: targeting and incapacitating cancer cells
Abstract
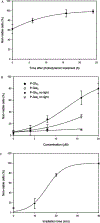
Since the role of saccharides in cell recognition, metabolism, and cell labeling is well-established, the conjugation of saccharides to drugs is an active area of research. Thus, one goal in the use of saccharide-drug conjugates is to impart a greater specificity toward a given cell type or other targets. Although widely used to treat some cancers and age related macular degeneration, the drugs used in photodynamic therapy (PDT) display poor chemical selectivity toward the intended targets, and uptake by cells most likely arises from passive, diffusional processes. Instead, the specific irradiation of the target tissues, and the formation of the toxic species in situ, are the primary factors that modulate the selectivity in the present mode of PDT. We report herein a two-step method to make nonhydrolyzable saccharide-porphyrin conjugates in high yields using a tetra(pentafluorophenyl)porphyrin and the thio derivative of the sugar. As a demonstration of their properties, the selective uptake (and/or binding) of these compounds to several cancer cell types was examined, followed by an investigation of their photodynamic properties. As expected, different malignant cell types take up one type of saccharide-porphyrin conjugate preferentially over others; for example, human breast cancer cells (MDA-MB-231) absorb a tetraglucose-porphyrin conjugate over the corresponding galactose derivative. Doseametric studies reveal that these saccharide-porphyrin conjugates exhibit varying PDT responses depending on drug concentration and irradiation energy. (1) Using 20 microM conjugate and greater irradiation energy induces cell death by necrosis. (2) When 10-20 microM conjugate and less irradiation energy are used, both necrosis and apoptosis are observed. (3) Using 10 microM and the least irradiation energy, a significant reduction in cell migration is observed, which indicates a reduction in aggressiveness of the cancer cells.
Figures






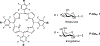

References
-
- MacDonald IJ, and Dougherty TJ (2001) Basic principles of photodynamic therapy, J. Porphyrins Phthalocyanines 5, 105–129.
-
- Neurath AR, Strick N, and Debnath AK (1995) Structural requirements for and consequences of an antiviral porphyrin binding to the V3 loop of the human immunodeficiency virus (HIV-1) envelope glycoprotein gp120, J. Mol. Recog 8, 345–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

