The external TEA binding site and C-type inactivation in voltage-gated potassium channels
- PMID: 15326027
- PMCID: PMC1304785
- DOI: 10.1529/biophysj.104.046664
The external TEA binding site and C-type inactivation in voltage-gated potassium channels
Abstract
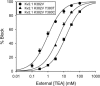
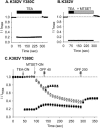
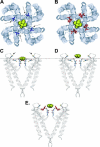
The location of the tetraethylammonium (TEA) binding site in the outer vestibule of K+ channels, and the mechanism by which external TEA slows C-type inactivation, have been considered well-understood. The prevailing model has been that TEA is coordinated by four amino acid side chains at the position equivalent to Shaker T449, and that TEA prevents a constriction that underlies inactivation via a foot-in-the-door mechanism at this same position. However, a growing body of evidence has suggested that this picture may not be entirely correct. In this study, we reexamined these two issues, using both the Kv2.1 and Shaker potassium channels. In contrast to results previously obtained with Shaker, substitution of the tyrosine at Kv2.1 position 380 (equivalent to Shaker 449) with a threonine or cysteine had a relatively minor effect on TEA potency. In both Kv2.1 and Shaker, modification of cysteines at position 380/449 by 2-(trimethylammonium)ethyl methanethiosulfonate (MTSET) proceeded at identical rates in the absence and presence of TEA. Additional experiments in Shaker demonstrated that TEA bound well to C-type inactivated channels, but did not interfere with MTSET modification of C449 in inactivated channels. Together, these findings rule out the possibility that TEA binding involves an intimate interaction with the four side chains at the position equivalent to Shaker 449. Moreover, these results argue against the model whereby TEA slows inactivation via a foot-in-the-door mechanism at position 449, and also argue against the hypothesis that the position 449 side chains move toward the center of the conduction pathway during inactivation. Occupancy by TEA completely prevented MTSET modification of a cysteine in the outer-vestibule turret (Kv2.1 position 356/Shaker position 425), which has been shown to interfere with both TEA binding and the interaction of K+ with an external binding site. Together, these data suggest that TEA is stabilized in a more external position in the outer vestibule, and does not bind via direct coordination with any specific outer-vestibule residues.
Figures








References
-
- Baukrowitz, T., and G. Yellen. 1996. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K+ channel. Science. 271:653–656. - PubMed
-
- Blaustein, R. O., P. A. Cole, C. Williams, and C. Miller. 2000. Tethered blockers as molecular ‘tape measures’ for a voltage-gated K+ channel. Nat. Struct. Biol. 7:309–311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

