Calcium-dependent regulation of the cell cycle via a novel MAPK--NF-kappaB pathway in Swiss 3T3 cells
- PMID: 15326199
- PMCID: PMC2172420
- DOI: 10.1083/jcb.200402136
Calcium-dependent regulation of the cell cycle via a novel MAPK--NF-kappaB pathway in Swiss 3T3 cells
Abstract
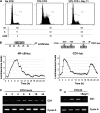
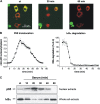
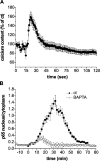
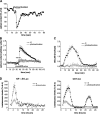
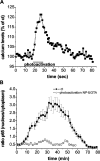
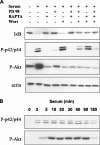
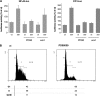
Nuclear factor kappa B (NF-kappaB) has been implicated in the regulation of cell proliferation and transformation. We investigated the role of the serum-induced intracellular calcium increase in the NF-kappaB--dependent cell cycle progression in Swiss 3T3 fibroblasts. Noninvasive photoactivation of a calcium chelator (Diazo-2) was used to specifically disrupt the transient rise in calcium induced by serum stimulation of starved Swiss 3T3 cells. The serum-induced intracellular calcium peak was essential for subsequent NF-kappaB activation (measured by real-time imaging of the dynamic p65 and IkappaBalpha fluorescent fusion proteins), cyclin D1 (CD1) promoter-directed transcription (measured by real-time luminescence imaging of CD1 promoter-directed firefly luciferase activity), and progression to cell division. We further showed that the serum-induced mitogen-activated protein kinase (MAPK) phosphorylation is calcium dependent. Inhibition of the MAPK- but not the PtdIns3K-dependent pathway inhibited NF-kappaB signaling, and further, CD1 transcription and cell cycle progression. These data suggest that a serum-dependent calcium signal regulates the cell cycle via a MAPK--NF-kappaB pathway in Swiss 3T3 cells.
Figures

Similar articles
-
Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1.Cancer Res. 2003 Aug 1;63(15):4375-83. Cancer Res. 2003. PMID: 12907607
-
Activation of phosphatidylinositol 3-kinase and c-Jun-N-terminal kinase cascades enhances NF-kappaB-dependent gene transcription in BCG-stimulated macrophages through promotion of p65/p300 binding.J Leukoc Biol. 2004 Apr;75(4):689-97. doi: 10.1189/jlb.0603280. Epub 2004 Jan 23. J Leukoc Biol. 2004. PMID: 14742634
-
Histone deacetylase inhibition down-regulates cyclin D1 transcription by inhibiting nuclear factor-kappaB/p65 DNA binding.Mol Cancer Res. 2005 Feb;3(2):100-9. doi: 10.1158/1541-7786.MCR-04-0070. Mol Cancer Res. 2005. PMID: 15755876
-
NF-kappaB and cell-cycle regulation: the cyclin connection.Cytokine Growth Factor Rev. 2001 Mar;12(1):73-90. doi: 10.1016/s1359-6101(00)00018-6. Cytokine Growth Factor Rev. 2001. PMID: 11312120 Review.
-
NF-kappaB signaling--an alternate pathway for oxidative stress responses.Cell Cycle. 2003 Jan-Feb;2(1):9-10. doi: 10.4161/cc.2.1.234. Cell Cycle. 2003. PMID: 12695674 Review. No abstract available.
Cited by
-
Store-operated CRAC channels regulate gene expression and proliferation in neural progenitor cells.J Neurosci. 2014 Jul 2;34(27):9107-23. doi: 10.1523/JNEUROSCI.0263-14.2014. J Neurosci. 2014. PMID: 24990931 Free PMC article.
-
Förster Resonance Energy Transfer-Based Single-Cell Imaging Reveals Piezo1-Induced Ca2+ Flux Mediates Membrane Ruffling and Cell Survival.Front Cell Dev Biol. 2022 May 13;10:865056. doi: 10.3389/fcell.2022.865056. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35646889 Free PMC article.
-
Routes of Ca2+ Shuttling during Ca2+ Oscillations: FOCUS ON THE ROLE OF MITOCHONDRIAL Ca2+ HANDLING AND CYTOSOLIC Ca2+ BUFFERS.J Biol Chem. 2015 Nov 20;290(47):28214-28230. doi: 10.1074/jbc.M115.663179. Epub 2015 Sep 22. J Biol Chem. 2015. PMID: 26396196 Free PMC article.
-
TRPV Protein Family-From Mechanosensing to Cancer Invasion.Biomolecules. 2021 Jul 13;11(7):1019. doi: 10.3390/biom11071019. Biomolecules. 2021. PMID: 34356643 Free PMC article. Review.
-
Targeting Orai1-mediated store-operated calcium entry by RP4010 for anti-tumor activity in esophagus squamous cell carcinoma.Cancer Lett. 2018 Sep 28;432:169-179. doi: 10.1016/j.canlet.2018.06.006. Epub 2018 Jun 15. Cancer Lett. 2018. PMID: 29908962 Free PMC article.
References
-
- Albanese, C., J. Johnson, G. Watanabe, N. Eklund, D. Vu, A. Arnold, and R.G. Pestell. 1995. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem. 270:23589–23597. - PubMed
-
- Baeuerle, P.A., and D. Baltimore. 1996. NF-κB: ten years after. Cell. 87:13–20. - PubMed
-
- Baldwin, A.S., Jr. 1996. The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649–683. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

