Identification of a quinone-sensitive redox switch in the ArcB sensor kinase
- PMID: 15326287
- PMCID: PMC516565
- DOI: 10.1073/pnas.0403064101
Identification of a quinone-sensitive redox switch in the ArcB sensor kinase
Abstract
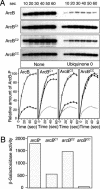
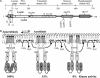
Escherichia coli senses and signals anoxic or low redox conditions in its growth environment by the Arc two-component system. Under anaerobic conditions, the ArcB sensor kinase autophosphorylates and transphosphorylates ArcA, a global transcriptional regulator that controls the expression of numerous operons involved in respiratory or fermentative metabolism. Under aerobic conditions, the kinase activity of ArcB is inhibited by the quinone electron carriers that act as direct negative signals. Here, we show that the molecular mechanism of kinase silencing involves the oxidation of two cytosol-located redox-active cysteine residues that participate in intermolecular disulfide bond formation, a reaction in which the quinones provide the source of oxidative power. Thus, a pivotal link in the Arc signal transduction pathway connecting the redox state of the quinone pool to the transcriptional apparatus is elucidated.
Figures

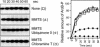

Similar articles
-
Ubiquinone and menaquinone electron carriers represent the yin and yang in the redox regulation of the ArcB sensor kinase.J Bacteriol. 2013 Jul;195(13):3054-61. doi: 10.1128/JB.00406-13. Epub 2013 May 3. J Bacteriol. 2013. PMID: 23645604 Free PMC article.
-
Quinones as the redox signal for the arc two-component system of bacteria.Science. 2001 Jun 22;292(5525):2314-6. doi: 10.1126/science.1059361. Science. 2001. PMID: 11423658
-
In vitro and in vivo analysis of the ArcB/A redox signaling pathway.Methods Enzymol. 2010;471:205-28. doi: 10.1016/S0076-6879(10)71012-0. Epub 2010 Mar 1. Methods Enzymol. 2010. PMID: 20946850
-
Signaling by the arc two-component system provides a link between the redox state of the quinone pool and gene expression.Antioxid Redox Signal. 2006 May-Jun;8(5-6):781-95. doi: 10.1089/ars.2006.8.781. Antioxid Redox Signal. 2006. PMID: 16771670 Review.
-
Cellular and molecular physiology of Escherichia coli in the adaptation to aerobic environments.J Biochem. 1996 Dec;120(6):1055-63. doi: 10.1093/oxfordjournals.jbchem.a021519. J Biochem. 1996. PMID: 9010748 Review.
Cited by
-
The sigmaS subunit of RNA polymerase as a signal integrator and network master regulator in the general stress response in Escherichia coli.Sci Prog. 2007;90(Pt 2-3):103-27. doi: 10.3184/003685007X215922. Sci Prog. 2007. PMID: 17725229 Free PMC article. Review.
-
Physiological roles of ArcA, Crp, and EtrA and their interactive control on aerobic and anaerobic respiration in Shewanella oneidensis.PLoS One. 2010 Dec 28;5(12):e15295. doi: 10.1371/journal.pone.0015295. PLoS One. 2010. PMID: 21203399 Free PMC article.
-
Diversity in Sensing and Signaling of Bacterial Sensor Histidine Kinases.Biomolecules. 2021 Oct 15;11(10):1524. doi: 10.3390/biom11101524. Biomolecules. 2021. PMID: 34680156 Free PMC article. Review.
-
The iron/heme regulated genes of Haemophilus influenzae: comparative transcriptional profiling as a tool to define the species core modulon.BMC Genomics. 2009 Jan 7;10:6. doi: 10.1186/1471-2164-10-6. BMC Genomics. 2009. PMID: 19128474 Free PMC article.
-
Impaired respiration elicits SrrAB-dependent programmed cell lysis and biofilm formation in Staphylococcus aureus.Elife. 2017 Feb 21;6:e23845. doi: 10.7554/eLife.23845. Elife. 2017. PMID: 28221135 Free PMC article.
References
-
- Parkinson, J. S. & Kofoid, E. C. (1992) Annu. Rev. Genet. 26, 71-112. - PubMed
-
- Hoch, J. A. & Silhavy, T. J. (1995) Two-Component Signal Transduction (Am. Soc. Microbiol., Washington, DC).
-
- Lynch, A. S. & Lin, E. C. (1996) in Escherichia coli and Salmonella: Cellular and Molecular Biology, eds. Neidhardt, F. C., Curtis, R., III, Ingraham, A. L., Lin, E. C. C., Low, K. B., Magasanik, B., Reznikoff, W. S., Riley, M., Schaechter, M. & Umbarger, H. E. (Am. Soc. Microbiol., Washington, DC), pp. 1526-1538.
-
- Iuchi, S., Matzuda, Z., Fujiwara, T. & Lin, E. C. (1990) Mol. Microbiol. 4, 715-727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

