Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein
- PMID: 15326293
- PMCID: PMC516468
- DOI: 10.1073/pnas.0404739101
Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein
Abstract
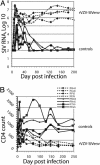
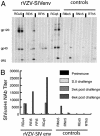
Given the dual role of CD4 T cells as both immune effectors and targets for HIV infection, the balance of CD4 versus CD8 T cell-mediated responses induced by candidate AIDS vaccines may be critical in determining postvaccination infection outcomes. An attenuated recombinant varicella-zoster virus vaccine expressing the simian immunodeficiency virus (SIV) envelope (Env) elicited nonneutralizing Env-binding antibodies and little if any cytotoxic T lymphocyte responses in rhesus macaques (Macaca mulatta). After challenge with SIV, Env vaccinees manifested increased levels of SIV replication, more rapid CD4 depletion, and accelerated progression to AIDS compared with controls. Enhanced SIV replication correlated with increased CD4 T cell proliferation soon after SIV challenge, apparently the result of an anamnestic response to SIV antigens. Thus activation of virus-specific CD4 T cells at the time of exposure to a CD4 T cell-tropic lentivirus, in the absence of an effective CD8 response, may enhance virus replication and disease. These data suggest suggest that candidate AIDS vaccines may not simply be either efficacious or neutral; they may also have the potential to be harmful.
Copyright 2004 The National Academy of Sciencs of the USA
Figures


References
-
- Douek, D. C., Brenchley, J. M., Betts, M. R., Ambrozak, D. R., Hill, B. J., Okamoto, Y., Casazza, J. P., Kuruppu, J., Kunstman, K., Wolinsky, S., et al. (2002) Nature 417, 95-98. - PubMed
-
- Krause, P. R. & Klinman, D. M. (2000) Nat. Med. 6, 451-454. - PubMed
-
- Institute of Laboratory Animal Resources, National Research Council (1996) Guide for the Care and Use of Laboratory Animals (Natl. Acad. Press, Washington, DC).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

