H2-rich fluids from serpentinization: geochemical and biotic implications
- PMID: 15326313
- PMCID: PMC516479
- DOI: 10.1073/pnas.0405289101
H2-rich fluids from serpentinization: geochemical and biotic implications
Abstract
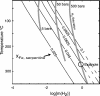
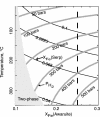
Metamorphic hydration and oxidation of ultramafic rocks produces serpentinites, composed of serpentine group minerals and varying amounts of brucite, magnetite, and/or FeNi alloys. These minerals buffer metamorphic fluids to extremely reducing conditions that are capable of producing hydrogen gas. Awaruite, FeNi3, forms early in this process when the serpentinite minerals are Fe-rich. Olivine with the current mantle Fe/Mg ratio was oxidized during serpentinization after the Moon-forming impact. This process formed some of the ferric iron in the Earth's mantle. For the rest of Earth's history, serpentinites covered only a small fraction of the Earth's surface but were an important prebiotic and biotic environment. Extant methanogens react H2 with CO2 to form methane. This is a likely habitable environment on large silicate planets. The catalytic properties of FeNi3 allow complex organic compounds to form within serpentinite and, when mixed with atmospherically produced complex organic matter and waters that circulated through basalts, constitutes an attractive prebiotic substrate. Conversely, inorganic catalysis of methane by FeNi3 competes with nascent and extant life.
Copyright 2004 The National Academy of Sciencs of the USA
Figures


References
-
- Dick, H. J. B., Lin, J. & Schouten, H. (2003) Nature 426, 405–412. - PubMed
-
- Hemley, J. J., Montoya, J. W., Shaw, D. R. & Luce, R. W. (1977) Am. J. Sci. 277, 353–383.
-
- Früh-Green, G. L., Connolly, J. A. D., Plas, A., Kelley, D. S. & Grobéty, B. (2004) AGU Mongr., in press.
-
- Abrajano, Y. A., Sturchio, N. C., Bohlke, J. K., Lyon, G. L., Pordea, R. J. & Stevens, C. M. (1988) Chem. Geol. 71, 211–222.
-
- Barnes, I., O'Neil, J. R. & Trescases, J. J. (1978) Geochim. Cosmochim. Acta 42, 144–145.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

