Vancomycin pharmacokinetics in critically ill patients receiving continuous venovenous haemodiafiltration
- PMID: 15327585
- PMCID: PMC1884563
- DOI: 10.1111/j.1365-2125.2004.02143.x
Vancomycin pharmacokinetics in critically ill patients receiving continuous venovenous haemodiafiltration
Abstract
Aims: To investigate the pharmacokinetics of vancomycin in critically ill patients on continuous venovenous haemodiafiltration (CVVHDF), a continuous renal replacement therapy (CRRT) and to see if routine measures approximate vancomycin clearance.
Methods: Pharmacokinetic profiles (15) of initial and steady-state doses of 750 mg twice daily intravenous vancomycin were obtained from blood and ultrafiltrate samples from 10 critically ill patients in the intensive care unit, with acute renal failure on CVVHDF (1 l h(-1) dialysate plus 2 l h(-1) filtration solution; 3 l h(-1) effluent; extracorporeal blood flow 200 ml min(-1)).
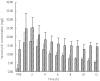
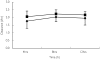
Results: CVVHDF clearance of vancomycin was 1.8 +/- 0.4 l h(-1) (30 +/- 6.7 ml min(-1)). This was 1.3-7.2 times that reported previously for vancomycin using other forms of CRRT. Total vancomycin body clearance was 2.5 +/- 0.7 l h(-1) (41.7 +/- 11.7 ml min(-1)). The clearance of vancomycin by CVVHDF was 76 +/- 16.5% of the total body clearance. CVVHDF removed approximately half the vancomycin dose during the 12-h period (A(CVVHDF) = 413 mg). The fraction eliminated by all routes was 60%. The sieving coefficient for vancomycin was 0.7 +/- 0.1 and for urea was 0.8 +/- 0.06.
Conclusions: Vancomycin is cleared effectively by CVVHDF. Clearance was faster than other forms of CRRT, therefore doses need to be relatively high. Urea clearance slightly overestimates vancomycin clearance. The administered doses of 750 mg every 12 h were too high and accumulation occurred, as only approximately 60% of a dose was cleared over this period. The maintenance dose required to achieve a target average steady-state plasma concentration of 15 mg l(-1) can be calculated as 450 mg every 12 h.
Figures
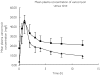
 ); Subsequent doses (Profile B only) or patients 1–4, 6, 7, 8, 9 and 10 (
); Subsequent doses (Profile B only) or patients 1–4, 6, 7, 8, 9 and 10 ( )
)
Similar articles
-
Vancomycin clearance during continuous venovenous haemofiltration in critically ill patients.Int J Antimicrob Agents. 2011 Aug;38(2):152-6. doi: 10.1016/j.ijantimicag.2011.04.010. Epub 2011 Jun 1. Int J Antimicrob Agents. 2011. PMID: 21636256
-
Pharmacokinetics of moxifloxacin in patients undergoing continuous venovenous haemodiafiltration.J Antimicrob Chemother. 2004 Oct;54(4):780-4. doi: 10.1093/jac/dkh421. Epub 2004 Sep 3. J Antimicrob Chemother. 2004. PMID: 15347636
-
Doripenem population pharmacokinetics and dosing requirements for critically ill patients receiving continuous venovenous haemodiafiltration.J Antimicrob Chemother. 2014 Sep;69(9):2508-16. doi: 10.1093/jac/dku177. Epub 2014 May 30. J Antimicrob Chemother. 2014. PMID: 24879665
-
[Continuous venovenous hemodiafiltration in critically ill patients with acute renal failure].Ugeskr Laeger. 2000 May 15;162(20):2868-71. Ugeskr Laeger. 2000. PMID: 10860424 Review. Danish.
-
Principles of antibacterial dosing in continuous renal replacement therapy.Crit Care Med. 2009 Jul;37(7):2268-82. doi: 10.1097/CCM.0b013e3181aab3d0. Crit Care Med. 2009. PMID: 19487930 Review.
Cited by
-
[Questionnaire surveying nephrologists on drug dose adjustment in patients with impaired kidney function].Wien Klin Wochenschr. 2010 Aug;122(15-16):479-85. doi: 10.1007/s00508-010-1421-2. Epub 2010 Aug 4. Wien Klin Wochenschr. 2010. PMID: 20683672 German.
-
Anti-infective Medicines Use in Children and Neonates With Pre-existing Kidney Dysfunction: A Systematic Review.Front Pediatr. 2022 Apr 26;10:868513. doi: 10.3389/fped.2022.868513. eCollection 2022. Front Pediatr. 2022. PMID: 35558367 Free PMC article.
-
Effect of therapeutic plasma exchange on amoxicillin, clindamycin, midazolam, and morphine pharmacokinetics in a critically ill child.Pediatr Nephrol. 2025 Jul 3. doi: 10.1007/s00467-025-06873-4. Online ahead of print. Pediatr Nephrol. 2025. PMID: 40608096
-
Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy.Clin Pharmacokinet. 2007;46(12):997-1038. doi: 10.2165/00003088-200746120-00003. Clin Pharmacokinet. 2007. PMID: 18027987 Review.
-
Antibiotic dosing in critically ill patients with acute kidney injury.Nat Rev Nephrol. 2011 Apr;7(4):226-35. doi: 10.1038/nrneph.2011.12. Epub 2011 Feb 22. Nat Rev Nephrol. 2011. PMID: 21343897 Review.
References
-
- Chow AW, Azar RM. Glycopeptides and nephrotoxicity. Intensive Care Med. 1994;20(Suppl 4):S23–9. - PubMed
-
- Gruneberg RN, Wilson AP. Anti-infective treatment in intensive care: the role of glycopeptides. Intensive Care Med. 1994;20(Suppl 4):S17–22. - PubMed
-
- Schetz M, Ferdinande P, Van den Berghe G, Verwaest C, Lauwers P. Pharmacokinetics of continuous renal replacement therapy. Intensive Care Med. 1995;21:612–20. - PubMed
-
- Bressolle F, Kinowski JM, de la Coussaye JE, Wynn N, Eledjam JJ, Galtier M. Clinical pharmacokinetics during continuous haemofiltration. Clin Pharmacokinet. 1994;26:457–71. - PubMed
-
- Cotterill S. Antimicrobial prescribing in patients on haemofiltration. J Antimicrob Chemother. 1995;36:773–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

