Penetration of Candida biofilms by antifungal agents
- PMID: 15328087
- PMCID: PMC514766
- DOI: 10.1128/AAC.48.9.3291-3297.2004
Penetration of Candida biofilms by antifungal agents
Abstract
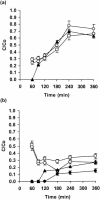
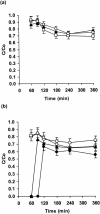
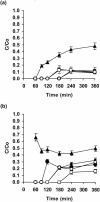
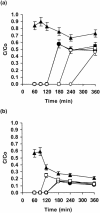
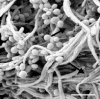
A filter disk assay was used to investigate the penetration of antifungal agents through biofilms containing single and mixed-species biofilms containing Candida. Fluconazole permeated all single-species Candida biofilms more rapidly than flucytosine. The rates of diffusion of either drug through biofilms of three strains of Candida albicans were similar. However, the rates of drug diffusion through biofilms of C. glabrata or C. krusei were faster than those through biofilms of C. parapsilosis or C. tropicalis. In all cases, after 3 to 6 h the drug concentration at the distal edge of the biofilm was very high (many times the MIC). Nevertheless, drug penetration failed to produce complete killing of biofilm cells. These results indicate that poor antifungal penetration is not a major drug resistance mechanism for Candida biofilms. The abilities of flucytosine, fluconazole, amphotericin B, and voriconazole to penetrate mixed-species biofilms containing C. albicans and Staphylococcus epidermidis (a slime-producing wild-type strain, RP62A, and a slime-negative mutant, M7) were also investigated. All four antifungal agents diffused very slowly through these mixed-species biofilms. In most cases, diffusion was slower with biofilms containing S. epidermidis RP62A, but amphotericin B penetrated biofilms containing the M7 mutant more slowly. However, the drug concentrations reaching the distal edges of the biofilms always substantially exceeded the MIC. Thus, although the presence of bacteria and bacterial matrix material undoubtedly retarded the diffusion of the antifungal agents, poor penetration does not account for the drug resistance of Candida biofilm cells, even in these mixed-species biofilms.
Figures

References
-
- Adam, B., G. S. Baillie, and L. J. Douglas. 2002. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 51:344-349. - PubMed
-
- Allison, D. G., and M. J. Matthews. 1992. Effect of polysaccharide interactions on antibiotic susceptibility of Pseudomonas aeruginosa. J. Appl. Bacteriol. 73:484-488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

